Laminopathies are an heterogeneous group of human disorders caused by mutations in the lamin A/C gene or in genes coding for lamin-binding proteins. They include several tissue-specific disorders (e.g., Emery-Dreifuss muscular distrophy and dilated cardiomyopathy with conduction defects) or systemic forms including the most severe phenotypes: Hutchinson-Gilford progeria syndrome (HGPS) and restrictive dermopathy (RD).Citation1 RD is a lethal neonatal laminopathy causing bone resorption of clavicles, tight translucent skin, anomalous facial features and arthrogryposis.Citation2 RD is a secondary laminopathy, since it is associated with mutations of a lamin-binding protein, the endoprotease ZMPSTE24 involved in lamin A precursor (prelamin A) post-translational processing.Citation3 A common mutation in the ZMPSTE24 gene (c.1085_1086InsT) has been characterized in most RD patients leading to impaired protein expression and prelamin A accumulation in cells.
Prelamin A processing consists of four steps: farnesylation of the C-terminal CaaX motif by the enzyme protein farnesyl-transferase, cleavage of the last three aminoacids by ZMPSTE24, carboxymethylation of the C-terminal cysteine by Icmt methyl-transferase and proteolytic removal of the last 18 aminoacids by ZMPSTE24. Thus, four processing intermediates are formed during prelamin A maturation.Citation4 Since also the RCE1 endoprotease catalyses the first cleavage step,Citation3 ZMPSTE24 absence should cause accumulation of farnesylated-carboxymethylated prelamin A.
Accumulation of different prelamin A forms causes different effects on nuclear organization:Citation5,Citation6 accumulating farnesylated prelamin A causes nuclear enlargement and mis-shapening, along with changes in the localization pattern of heterochromatin-associated proteins HP1 and tri-H3K9,Citation7 while accumulating non-farnesylated prelamin A produces intranuclear prelamin A and heterochromatin clusters, with re-localization of heterochromatin markers, without nuclear enlargement.Citation5 Also, impaired lamin maturation promotes DNA damage accumulation.Citation8,Citation9
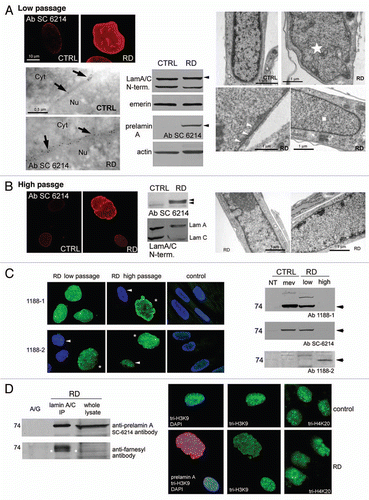
We analyzed prelamin A processing in four different RD cell lines bearing the same ZMPSTE24 mutationCitation2 using an antibody (Sc-6214) directed to a C-terminal epitope of prelamin A.Citation10 The experiments were performed at low (5–12) and at high (16–22) culture passage numbers.
Low passage RD cells showed high prelamin A levels, but undetectable mature lamin A; prelamin A was localized exclusively at the nuclear rim by both immunofluorescence and colloidal gold-immunoelectron microscopy; western blot analysis confirmed this data (part A). TEM analysis showed severe nuclear morphology defects (enlarged size, envelope invaginations) and heterochromatin disorganization (areas devoid of heterochromatin) in 10–30% of cells, while most of RD nuclei were normally shaped, though presented nuclear envelope duplications and severe heterochromatin loss (part A). Surprisingly, at high passage numbers, labeling by Sc-6214 was reduced in about 90% of nuclei and undetectable in several cells; only highly dysmorphic nuclei showed intense prelamin A staining. Ultrastructural analysis of high passage fibroblasts revealed improved peripheral heterochromatin organization in 50% of the normally shaped nuclei and peripheral heterochromatin clumps in 5% of nuclei. At this stage (p.16–22), western blot analysis revealed prelamin A as a doublet (part B).
To characterize prelamin A forms, we used selective antibodies: 1188-1 (directed to the full-length prelamin A-specific C-terminus sequence) detects both non-farnesylated and farnesylated prelamin A, provided that the CSIM terminal sequence is maintained; 1188-2 (directed to the farnesylated prelamin A-specific C-terminus lacking the SIM sequence) detects farnesylated-carboxymethylated prelamin A.Citation4
1188-1 revealed intense fluorescence at low passage number in both normally shaped and dysmorphic nuclei indicating high levels of full-length prelamin A accumulation, while 1188-2 labelled only dysmorphic nuclei, suggesting that farnesylated-carboxymethylated prelamin A was less represented.
At high passage numbers, 1188-2 labelled the normally shaped nuclei, while both antibodies revealed intense fluorescence in highly dysmorphic enlarged nuclei (part C). 1188-1 and SC-6214, but not 1188-2, revealed the prelamin A band in western blot analysis at low passages, confirming the small amount of farnesylated-carboxymethylated prelamin A (non-farnesylated, full-length prelamin A-accumulating control was obtained with MevinolinCitation11). On the contrary, 1188-2 revealed a faint band in very high passage (p.25) cells, which did not accumulate the prelamin A form(s) detectable by 1188-1 or Sc-6214 (part C).
We then immunoprecipitated the whole prelamin A exploiting an anti-lamin A/C N-terminus antibody and revealed the immunoprecipitated bands with anti-prelamin A or anti-farnesyl antibodies confirming farnesylation of prelamin A bands (panel D).
Searching for functional defects in RD nuclei, we labeled prelamin A and the heterochromatin marker tri-H3K9 or tri-H4K20. At any passage, dysmorphic nuclei accumulating both prelamin A forms, showed altered distribution (i.e., without clusters) or loss (in some nuclei) of tri-H3K9 fluorescence and disorganization of heterochromatic areas (part D); normally shaped nuclei accumulating prelamin A, showed mostly unaffected tri-H3K9, which was only slightly more clustered in a low percentage of nuclei. Analogous alterations were revealed by tri-H4K20 labelling.
In conclusion, we detected significant changes in prelamin A levels and post-translational modifications in all cell lines, depending on the passage number. So far, increased prelamin A levels have been observed in progeroid laminopathies, depending on the passage number or on the patient's age.Citation7,Citation12 We show that accumulation of full-length, farnesylated protein form prevails in RD fibroblasts, and that a subpopulation of cells accumulates high levels of both full-length and farnesylated-carboxymethylated prelamin A. Finally, we demonstrate that accumulation of both forms is associated with the most severe abnormalities, i.e., nuclear enlargement and mis-shaping and severe chromatin defects. Our data suggest that the first cleavage step in prelamin A post-translational processing is mostly carried out by the ZMPSTE24 endoprotease, which is not efficiently replaced by other enzyme(s) in RD cells. However, our results suggest activation of alternative endoproteolytic processes, probably when high prelamin A levels are reached.
Figures and Tables
Figure 1 Immunochemical analysis of restrictive dermopathy fibroblasts. (A) Low passage cells. CTRL: control; RD: restrictive dermopathy; TEM immunogold labelling, Cyt: cytoplasm; Nu: nucleus; black arrows: gold granules on nuclear lamina; western blot, black arrowheads: prelamin A bands; TEM morphology, white star: dysmorphic nucleus; white arrowheads: nuclear envelope duplications; white square: heterochromatin loss. (B) High passage cells. Immunofluorescence, CTRL: control; RD: restrictive dermopathy; western blot, black arrowheads: prelamin A doublet; TEM morphology: heterochromatin recovery in RD. (C) Immunofluorescence; white arrowheads: normally shaped nuclei; white asterisks: dysmorphic nuclei; western blot, CTRL: control; RD: restrictive dermopathy; NT: not treated with mevinolin; mev: treated with mevinolin; low: low passage cells; high: high passage cells; black arrows: prelamin A bands. (D) IP: immunoprecipitation; white asterisks: prelamin A band position, overlapped by anti-farnesyl antibody; immunofluorescence, RD: restrictive dermopathy. All immunofluorescence pictures are taken at the same magnification.
Acknowledgements
This work was supported by the Italian Istituto Superiore di Sanità ‘Rare Diseases Italy-USA program’ [grant number 526/D30], Prin 2008 to G.L., the ‘Fondazione Carisbo’, Italy and A.I.Pro.Sa.B., Italy.
References
- Worman H, et al. Cold Spring Harb Perspect Biol 2010; 2:760
- Navarro C, et al. Hum Mol Genet 2005; 14:1503 - 1513
- Barrowman J, et al. Biol Chem 2009; 390:761 - 773
- Dominici S, et al. Eur J Histochem 2009; 53:43 - 52
- Lattanzi G, et al. J Cell Biochem 2007; 102:1149 - 1159
- Mattioli E, et al. Exp Cell Res 2008; 314:453 - 462
- Filesi I, et al. Physiol Genomics 2005; 23:150 - 158
- Liu Y, et al. J Cell Sci 2006; 119:4644 - 4649
- Liu Y, et al. FASEB J 2008; 22:603 - 611
- Gruber J, et al. J Cell Sci 2005; 118:689 - 696
- Columbaro M, et al. Cell Mol Life Sci 2005; 62:2669 - 2678
- Goldman R, et al. Proc Natl Acad Sci USA 2004; 101:8963 - 8968