Abstract
We recently described a new adhesion pathway in lymphocytes that is dependent on Cyclin-dependent kinase (Cdk) 4 activity and mediates lymphocyte interactions with endothelial matrix. We showed that Cdk4-/- mice had impaired recruitment of lymphocytes following bleomycin model of acute lung injury. In this study, we characterized the development and function of hematopoietic cells in Cdk4-/- mice and assessed the response of Cdk4-/- mice to allergen challenge. Cdk4-/- mice had hypoplastic thymuses with decreased total thymocyte cell numbers and increased CD4/CD8 double negative cells. Cdk4-/- bone marrow (BM) chimeric mice showed similar findings. Thymocytes from either Cdk4-/- or Cdk4-/- BM chimeric mice proliferated equally well as wild type controls in response to IL-2 activation. However Cdk4-/- thymocytes had decreased adhesion to both endothelial cell matrix and fibronectin compared to wildtype (WT) controls, whereas Cdk4-/- and WT splenocytes had similar adhesion. When Cdk4-/- BM chimeric mice and wild type BM chimeric mice were sensitized and challenged by intranasal administration of ovalbumin, we found no differences in allergic responses in the lung and airways between the two groups, as measured by inflammatory cell infiltrate, airway hyperreactivity, IgE levels and cytokine levels. In summary, we show that Cdk4 plays a previously unrecognized role in thymocyte maturation and adhesion, but is not required for thymocyte proliferation. In addition, Cdk4 is not required for lymphocyte trafficking to the lung following allergen sensitization and challenge.
Introduction
Cyclin-dependent kinases (Cdks) are Serine/Threonine kinases that regulate progression through the cell cycle. Cdk4 and Cdk6 act early in the cell cycle and are involved in the transition from G1 to S phase. In addition to their role in cell cycle, there is increasing evidence that Cdks, as well as cyclins and cyclindependent kinase inhibitors are important for other cellular functions, including cytoskeleton rearrangement and cell migration.Citation1 Despite its integral role in cell cycle progression, Cdk4−/− mice are viable, although the mice are infertile and small. Some of the mice have abnormalities in hypothalamic-pituitary axisCitation2,Citation3 and develop diabetes due to abnormal pancreatic islet cell formation.Citation4 Several members of the cell cycle family, including Cdk6,Citation5 and Cyclin D3,Citation6 have defects in hematopoietic development. However, the role of Cdk4 in lymphocyte development and impact on hematopoiesis has not previously been reported.
We recently demonstrated a role for Cdk4 in lymphocyte adhesion to and migration through endothelial cell matrix.Citation7 Furthermore, we showed that Cdk4−/− mice had impaired recruitment of lymphocytes in bronchoalveolar lavage following bleomycin-induced acute lung injury.Citation7 Lymphocyte trafficking to the lung is a key feature of the initial allergen response.Citation8 Once lymphocytes have crossed the endothelium and interact with the subendothelial tissue, additional signals guide lymphocytes into other compartments in the lung, such as the alveolar airspaces or the bronchial lumina.Citation9 To test whether Cdk4 pathway plays a role in this secondary trafficking of lymphocytes to the lung, we compared the allergen responses of CDK4−/− and wild-type (WT) bone marrow (bm) chimeric mice.
Results
Cdk4−/− mice have increased CD4/CD8 double negative thymocytes.
Since mice deficient in several components of cell cycle proteins have defects in lymphocytes subsets, we performed a comprehensive analysis of the cell populations within the hematopoietic compartments (thymus, peripheral blood, spleen, bone marrow) in Cdk4−/− and wild-type littermates by flow cytometry. As previously reported, Cdk4−/− were smaller than wild-type littermates (approximately 50% reduction in weight).Citation2 We found that the Cdk4−/− mice had hypoplastic thymuses, compared to wild-type littermates, even when adjusted for overall lower body weight, due to decreased thymocyte cell number (wild-type 72.51 ± 20.18 × 106 vs. Cdk4−/− 15.75 ± 11.88 × 106 cells) ().
In contrast, spleen size was comparable to wild-type littermates after adjustment for smaller body size. When we analyzed the thymic cell populations, we found that Cdk4−/− mice had an increase in CD4/CD8 double negative thymocytes, consistent with delayed (but not absent) maturation of lymphocytes (). There were no consistent differences in spleen, bone marrow or peripheral blood cell count and differential between wild type and Cdk4−/− mice (data not shown).
Since Cdk4−/− mice develop diabetes due to islet cell abnormalities,Citation2 we wanted to minimize the possibility that chronic illness contributed to thymic hypoplasia. Therefore, we developed hematopoietic chimeric mice and analyzed thymic cell populations after repopulation. We did not see any complications following total body irradiation in any of the chimeric mice. Wild-type (wt) mice transplanted with either wt or Cdk4−/− bone marrow (bm) displayed similar repopulation of spleen, lymph nodes and peripheral blood. Consistent with our analysis of Cdk4−/− mice, Cdk4−/− bm→wt mice (Cdk4−/− bm chimeric mice) had thymic hypoplasia and increased CD4/CD8 double negative thymocytes (). This is consistent with a primary defect in T cell maturation, rather than a secondary effect of chronic illness (i.e., diabetes in Cdk4−/− mice) on T cell maturation.
Cdk4−/− thymocytes, but not splenocytes, have impaired adhesion.
We previously demonstrated that Cdk4 played a role in unstimulated adhesion of lymphocytes to high density matrix or endothelial cell (EC)-matrix.Citation7 We asked whether cells derived from Cdk4−/− mice recapitulated this phenotype. We isolated thymocytes and splenocytes from Cdk4−/− mice and tested their adhesion to EC matrix or high-density fibronectin. We found that Cdk4−/− thymocytes had decreased adhesion to both EC-matrix and fibronectin compared to wt controls, whereas Cdk4−/− splenocytes had similar adhesion as wt splenocytes ( and B). T lymphocytes isolated from spleens demonstrated similar adhesion in both groups of mice (Fig. 3c). Since cyclin D3 is the major cyclin partner for Cdk4 in lymphocytes, we asked whether thymocytes derived from cyclin D3−/− had a similar adhesion defect. We found that cyclin D3−/− thymocytes also had decreased adhesion to fibronectin compared to wt thymocytes, but cyclin D3−/− splenocytes adhered equally well as wt splenocytes (), recapitulating our findings using Cdk4−/− cells.
Cdk4−/− thymocytes are able to proliferate and secrete cytokines.
We then asked if the previously described defect in lymphocyte recruitment to the lung in Cdk4−/− miceCitation7 was due to impaired proliferation of lymphocytes. Since Cdk4 is a key regulator of cell cycle progression, we examined the ability of cells to proliferate in response to either IL-2 (thymocytes) or CD3 (splenocytes). We found that thymocytes from either Cdk4−/− or Cdk4−/− bm chimeric mice proliferated equally well as wild type controls in response to IL-2 activation ( and not shown). Likewise, splenocytes obtained from Cdk4−/− or Cdk4−/− BM chimeric mice proliferated equally well as wild-type controls in response to CD3 activation (). In addition, CD3-stimulated lymphocytes from wt and Cdk4−/− mice had similar cytokine secretion profiles including secretion of IL-2 (). Therefore, the defect in lymphocyte accumulation is unlikely to be due to simply a defect in lymphocyte proliferation.
Lack of Cdk4 does not affect allergen-induced inflammation.
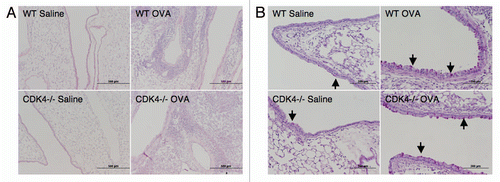
Lymphocyte trafficking to the lung is a key feature of the initial allergen response.Citation8 We previously demonstrated impaired lymphocyte recruitment to the lung in a short-term model of acute lung injury in Cdk4−/− mice.Citation7 To expand our previous findings, Cdk4−/− bm chimeric mice and appropriate control mice were sensitized and then challenged by intranasal administration of ovalbumin to produce an allergic response in the lung and airways ().Citation10,Citation11 We then assessed recruitment of inflammatory cells to the airspaces by bronchoalveolar lavage (BAL). As expected, OVA-sensitized and -challenged mice developed dramatic increases in total cell number with increased numbers of lymphocytes, eosinophils, neutrophils and macrophages. Despite previous demonstration of impaired adhesion and lymphocyte trafficking in Cdk4−/− mice,Citation7 there were no statistically significant differences in either the absolute number of lymphocytes or the percentage of lymphocytes in BAL cell differential in wild-type and Cdk4−/− bone marrow chimeric mice (). H&E staining of lungs from OVA-sensitized and -challenged mice confirmed the presence of a robust inflammatory response in wild-type and Cdk4−/− bone marrow chimeric mice (). Typical pathologic changes, including widespread patchy inflammatory infiltrates (mainly eosinophils, lymphocytes and alveolar macrophages) in the peribronchial and perivascular areas, were observed in lungs from both OVA challenged groups. These responses did not differ between Cdk4−/− bm chimeric mice and controls. In addition, we analyzed PAS-stained sections of lungs for mucus content. Saline-challenged mice had little if any mucus visible in the airways. OVA-challenged mice produced substantial amounts of mucus. However, no clear differences were observed between wild-type and Cdk4−/− bm chimeric mice (
Absence of hematopoietic Cdk4 does not affect baseline or allergen-induced airway hyperreactivity.
We asked whether airway reactivity (which correlates with lymphocyte recruitment) was altered in the Cdk4−/− bm chimeric mice following short-term allergen exposure. There were no differences in baseline airway reactivity between wild-type and Cdk4−/− bm chimeric mice. Following OVA sensitization and challenge, there is clear OVA-induced airway hyperreactivity in both groups, but no difference between control and Cdk4−/− bm chimeric mice ().
Lack of Cdk4 does not impair allergen-induced IgE production.
To determine whether Cdk4 contributes to the allergic humeral response, we measured serum OVA-specific IgE levels (). As expected, there was no measurable OVA-specific IgE in either group at baseline. OVA administration increased IgE serum levels in both wt and CDK4−/− bm chimeric mice and there were no differences between the two groups ().
Lack of Cdk4 does not affect cytokine expression in the lung.
Cytokine levels were measured in lung bronchoalveolar lavage fluid (). There were no differences in cytokine levels between wild-type and Cdk4−/− bm mice in saline treated mice. Following ovalbumin challenge, there were significant increases in IL-4, IL-5, IL-6 and IL-13. However, there were no differences detected between wt and Cdk4−/− bm mice. In addition, low levels of GM-CSF, IL-2, IL-10, IL-17, INFγ were measured at baseline, which did not change following ovalbumin challenge and were similar in all groups of mice (not shown).
Discussion
We analyzed the hematopoietic profile of Cdk4−/− mice. The most striking and consistent findings were hypoplastic thymuses and an increase in immature thymocytes, specifically CD4/CD8 double negative thymocytes. This phenotype was replicated in Cdk4−/− bm chimeric mice, demonstrating that the findings were not due to other systemic factors, such as chronic disease. Despite the effect on lymphocyte development, other hematopoietic components did not demonstrate consistent differences compared to wild-type mice.
Cdk4 binds to D-type cyclins (cyclin D1, D2, D3). In T lymphocytes, the primary cyclin D at baseline is cyclin D3 (reviewed in ref. Citation12 and data not shown). The cyclin D3 knockout mice are viable, fertile and appear overtly normal at baseline.Citation6 Cyclin D3−/− mice have a block during development of immature T lymphocytes, with an increase in CD4/CD8 double negative thymocytes.Citation6 In addition, we observed the same adhesion defect in cyclin D3−/− thymocytes as in Cdk4−/− thymocytes. Thus, the phenotype of Cdk4−/− mice in thymus and lymphocytes most closely resembles cyclin D3−/− mice, consistent with data demonstrating cyclin D3 is a major partner for Cdk4 in lymphocytes. In addition, cyclin D3 mice are less susceptible to the development of subsets of T cell malignancies.Citation6 While we did not assess the role of Cdk4 in malignancy development, we speculate that loss of Cdk4 would result in similar resistance.
During cell cycle progression, Cdk4 and Cdk6 act during the same phase of the cell cycle. Similar to Cdk4−/− mice, Cdk6−/− mice have hypoplastic thymuses with decreased cell numbers.Citation5 In contrast to Cdk4−/− mice, analysis of thymocyte development demonstrated an increase in single positive CD4 and CD8 lymphocytes, with concomitant decrease in double positive cells.Citation5 In addition, Cdk6−/− mice also had decreased spleen size and cellularity, decreased numbers of megakaryocytes and decreased cells of erythroid lineage.Citation5 Loss of both Cdk4 and Cdk6 results in embryonic lethality (although organogenesis still proceeds),Citation5 suggesting that Cdk4 and Cdk6 may each be able to compensate partially for the loss of the other Cdk. However, the differences in phenotype between these knockouts suggests that they are not entirely redundant and have distinct functions.
Our data support a role for Cdk4 in thymocyte development and in thymocyte adhesion. Interestingly, only thymocyte adhesion appeared to be affected, as Cdk4−/− splenocytes adhered similarly to wild-type. Since B lymphocytes represent the majority of splenocytes, we isolated T lymphocytes from spleens and confirmed results obtained with total splenocytes. This data suggests that as T lymphocytes mature, they loss dependence on Cdk4 for adhesion. Previous reports demonstrated that a majority of CD4/CD8 double positive thymocytes are able to adhere to fibronectin in the absence of activation.Citation13 This adhesion was mediated by the integrin α4β1.Citation13 We previously demonstrated that Cdk4-mediated adhesion to fibronectin was also primarily dependent on α4β1.Citation7 Thus, our data are consistent with Cdk4 playing a role in the constitutive adhesion of thymocytes to fibronectin using α4β1. Fibronectin is highly expressed in the thymic medulla, but has much lower expression in the cortex, establishing matrix gradients for thymocyte migration.Citation14 Since T cell development in the thymus requires migration from cortex into FN-rich medulla,Citation14 we speculate that lack of Cdk4 is impairing the ability of thymocytes to migrate and adhere into the medulla, thus affecting development of CD4/CD8 double positive thymocytes.
In allergic asthma, abnormal T cell accumulation in the lung contributes to the inappropriate inflammatory response.Citation15 The severity of airway hyperreactivity correlates with the number of T lymphocytes and eosinophils in the airways. The mechanisms directing trafficking through the different compartments in the lung are still not fully understood. Once lymphocytes have crossed the endothelium and interact with the subendothelial tissue, additional signals guide lymphocytes into other compartments in the lung, such as the alveolar airspaces or the bronchial lumina.Citation9 We initially hypothesized that Cdk4 pathway may play a role in this secondary trafficking. However, our results indicate that Cdk4 is not necessary for lymphocyte or eosinophil recruitment to the lung following allergen challenge, despite our previous data demonstrating impaired lymphocyte recruitment to the lung in bleomycin model of acute lung injury. One explanation is that the initial observed differences in lymphocyte recruitment were due to requirement of Cdk4 in structural cells rather than hematopoietic cells. Because of the multiple co-morbidities of the Cdk4−/− mice, we were not able to obtain wt bm→Cdk4−/− mice. However, we repeated bleomycin lung injury in BM chimeric mice and found similar decreases in lymphocyte cell count in Cdk4−/− bm→wt mice (data not shown). Since the phenotype from Cdk4−/− bm→wt mice is similar to our results from Cdk4−/− mice, that suggests the defect is due to hematopoietic cell expression of Cdk4 rather than lung parenchymal cell expression. In addition to differences in model systems, another differences is time points examined. Our initial data demonstrate a difference at day 3 following initial lung injury. The current experimental design examines mice at later time points after challenge. Since our in vitro data demonstrated that blockade of Cdk4 did not alter PMA-stimulated adhesion,Citation7 other adhesion pathways in lymphocytes will still be functional despite lack of Cdk4. Time course experiments may reveal more subtle abnormalities in lymphocyte recruitment to the lung in Cdk4−/− mice.
In summary, we demonstrate that lack of Cdk4 results in selective thymus hypoplasia, delayed maturation of thymocytes and an associated adhesion defect in thymocytes. However, lack of Cdk4 does not impair stimulated lymphocyte proliferation, cytokine production or allergen-induced lymphocyte recruitment to the lung.
Methods
Generation of chimeric mice.
This animal experiment was approved by the Institutional Animal Care and Use Committee of the University of Washington. Cdk4−/− miceCitation4 were backcrossed into C57Bl6 background for at least 8 generations. Cdk4+/+ littermates (“wild-types,” wt) were used as controls. Standard PCR was used to test genotype from mouse tail DNA or mouse spleen. PCR primers SW39 (5′-ATA TTG CTG AAG AGC TTG GCG G), HK327 (5′-CCA GCC TGA AGC TAA GAG TAG CTG T) and HK326 (5′-CGG AAG GCA GAG ATT CGC TTA T) were used to detect the Cdk4-null allele as a 315-bp fragment or wild-type as a 195 bp fragment. Cyclin D3−/− mice were a generous gift from Dr. Piotr Sicinski (Harvard Medical School, Boston, MA) and have been previously characterized in reference Citation6.
To generate bone marrow chimeric mice, hematopoietic bone marrow stem cells (3–8 million cells per recipient) from Cdk4+/+ or Cdk4−/− mice were injected via orbital sinus into 8-week old host C57Bl/6 mice (Jackson Laboratory, Bar Harbor, ME) that were lethally irradiated using an 11 Gray dose (1,100 rads) to eliminate their hematopoietic stem cells. Mice were allowed to recover and leukocytes reconstituted over 90 days. Engraftment was confirmed by PCR and western blot analysis of spleen lysates.
Monoclonal antibodies.
Anti-CD3e (clone 145-2C11), anti-CD4 (GK1.5), anti-CD8α (53.67), anti-B220 (RA3-6B2), anti-CD45 (30-F11), anti-CD11b (M1/70), anti-Ly-6G (RB6-8C5), anti-CD115 (AFS98) and anti-F4/80 (BM8) were obtained from eBioscience (San Diego, CA). These antibodies were directly conjugated to PE, FITC or PE-Cy5.
Cell staining and flow cytometry analysis.
Leukocytes from peripheral blood, spleen, bone marrow and thymus were treated with ammonium chloride buffer (150 mM NH4Cl, 10 mM KHCO3, 0.1 mM Na2EDTA) to lyse the red blood cells and then washed with FACS buffer (PBS with 0.2% BSA). Approximately 5 × 105 cells in a 200 µL volume were incubated with specific antibodies for 15 minutes at 4°C in the dark. Purified antimouse CD16/32 (Fcγ) antibody was present in each stained and unstained well to block nonspecific binding. Flow cytometric analyses were performed on a Guava EasyCyte Plus System (Millipore, Hayward, CA).
Cell adhesion assay.
Thymocytes or splenocytes were labeled with 2.5 µM calcein-AM (Molecular Probes, Eugene, OR) at room temperature for 20–40 min, washed and resuspended in HBSS with calcium and magnesium (Mediatech) supplemented with 0.1% BSA and 4 mM HEPES. In some experiments, T-lymphocytes were isolated from total splenocytes by negative selection using mouse T cell Isolation Kit (Miltenyi Biotec, Auburn, CA). Enrichment of T lymphocytes was confirmed by flow cytometry analysis with anti-CD4 antibody. After cells were allowed to adhere to endothelial cell derived matrix or fibronectin-coated wells for 15–30 min at 37°C, non-adherent cells were removed by washing x2. Adhesion was measured using a Cytofluor Series 4000 fluorescence plate reader (PerSeptive Biosystems, Framingham, MA). Plates were scanned before and after washing for total and adherent cells, respectively.
Cell proliferation assay.
Splenocytes or thymocytes were isolated from age-matched littermates. 2 × 105 thymocytes were plated into 96-well tissue culture plates in complete RPMI-1640 in the presence or absence of 500 U/mL IL-2 (Roche Applied Science) and 250 U/mL TNFα (R&D Systems, Minneapolis, MN) and incubated for four days. Splenocytes (2 × 105 cells per well) were grown in 96-well tissue culture plates pretreated with PBS or anti-mouse CD3e antibody (clone 145-2C11, eBioscience) for 72 h. Cells were then labeled with 10 µM BrdU for 20 hours and BrdU incorporation was measured with an anti-BrdU immunoassay (Roche Applied Science, Mannheim, Germany). Conditioned media was collected for cytokine measurement.
Ovalbumin challenge and airway response measurements.
Age-matched male chimeric mice were sensitized by intraperitoneal injections of saline or ovalbumin (OVA) (250 mg/ml) mixed with Alum (5 mg/ml) on day 0, 7 and 14, and then challenged by intranasal administration of saline or OVA (100 µg) on days 21, 22 and 23. On day 24, mice were anesthetized and ventilated with 100% oxygen by a Scireq Flexivent pulmonary mechanics analyzer at a tidal volume of 9 ml/kg and a respiratory rate of 150 breaths/min. Mice were paralyzed with pancuronium to minimize input from chest wall contraction. Acetylcholine concentration response curves were performed by intravenous injection of increasing concentrations of acetylcholine (0.03 to 3.0 µg/g body weight) at 3 minute intervals. Pulmonary resistance was measured every 3 seconds. Resistance was determined using a linear single compartment model. Airway pressure was measured at the tip of the tracheostomy tube and pressure was measured within the ventilator by SC-24 pressure transducers.
OVA-specific IgE assay.
Sera were obtained from blood collected by cardiac puncture from antigen- or vehicle-treated mice after airway responsiveness measurements. OVA-specific IgE levels were measured by ELISA using microplates coated with OVA. Diluted serum samples were added to each well. The bound IgE was detected with biotinylated anti-mouse IgE (R35-118; Pharmingen) and processed as previously described in reference Citation11. The results were expressed as OD450 nm values.
Assessment of pulmonary inflammation and mucus production.
Lungs underwent lavage five times with 0.8 ml of PBS. After centrifugation, the cell pellet was resuspended in normal saline after lysis of red blood cells. Total cells were counted with a hemacytometer. Cytospin preparations were prepared and stained with a HEMA 3 stain set (Fisher) and bronchoalveolar lavage fluid (BALF) cell differential percentages were determined based on light microscopic evaluation of > 300 cells/slide.
After lavage, lungs were inflated with 10% buffered formalin to 25 cm H2O of pressure, fixed with 10% buffered formalin and paraffin embedded. Sections (5 mm thick) were stained with H&E for standard morphology and with periodic acid-Schiff (PAS) for evaluation of mucus production.
Cytokine measurements.
Measurement of cytokines in BALF samples or conditioned media was performed using the Fluorokine® MultiAnalyte Profiling (MAP) Multiplex Mouse Cytokine Panel from R&D Systems (Minneapolis, MN USA). In brief, 50 µL of sample or standard were incubated with antibody-coated microspheres, washed and then incubated with biotinylated cytokine antibodies, followed by incubation with PE-labeled streptavidin. The samples were read by the Luminex® 100™ analyzer (BioRad). All samples were run in duplicate. The concentrations of cytokines were extrapolated from a standard curve for each protein.
Statistical analysis.
For normally distributed data, we used Student t-test to look for differences in mean of samples. Means of more than two groups of data were compared using ANOVA for analysis of one independent variable or two-way ANOVA, for analysis of two independent variables, followed by Tukey's honestly significant difference (HSD) post hoc test. Statistical significance was set at p < 0.05, using two-tailed test.
Abbreviations
| Cdk | = | cyclin-dependent kinase |
| BM | = | bone marrow |
| WT | = | wildtype |
| OVA | = | ovalbumin |
| BALF | = | bronchoalveolar lavage fluid |
| PAS | = | periodic acid-schiff |
| HSD | = | honestly significant difference |
| EC | = | endothelial cell |
| FN | = | fibronectin |
| BW | = | body weight |
Figures and Tables
Figure 1 Cdk4−/− mice have hypoplastic thymuses. (A) Thymus weight (mg) of wt and Cdk4−/− mice. Inset: representative image of thymuses. (B) Total thymocyte cell number of wt and Cdk4−/− mice. Each point represents an individual mouse. Mean ± SEM are shown.
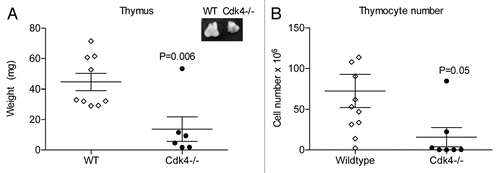
Figure 2 Cdk4−/− mice have increased CD4/CD8 double negative thymocytes. (A) Representative scatter plots of thymocytes from wt and Cdk4−/− mice (top) and wt or Cdk4−/− bm chimeras (bottom) stained with anti-CD8 (y-axis) and anti-CD4 (x-axis). (B) Percentage of CD4/CD8 double negative thymocytes. Top: n = 6/group, Bottom: bm chimeras n = 2/group. Means ± SD are shown. *p < 0.05. (C) Summary of flow cytometric analysis of thymocyte subsets.
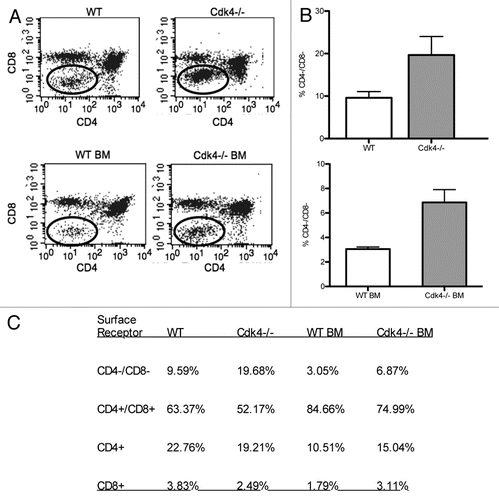
Figure 3 Cdk4−/− thymocytes have decreased adhesion. Primary isolated thymocytes (A), splenocytes (B) or or Cd4+ enriched splenocytes (C) derived from Cdk4−/− mice, cyclin D3−/− mice (D) or wild-type littermates were allowed to adhere to endothelial cell (EC)-matrix or 5 µg/ml fibronectin (FN) for 30 minutes. N = 3/group. Means ± SD are shown.
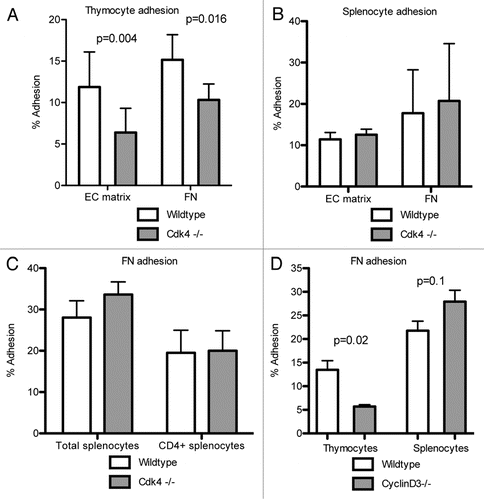
Figure 4 Cdk4−/− cells proliferate, secrete cytokines in response to stimulation. (A) Thymocytes were incubated for 48 h with IL-2. (B) Splenocytes were incubated for 48 h on anti-CD3 antibodies. Proliferation was measured by BrdU incorporation. (C) Cytokine expression in conditioned media from stimulated splenocytes. N = 3/group. Means ± SD are shown.
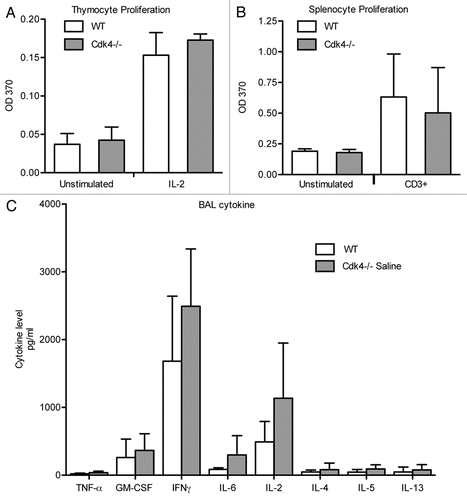
Figure 5 Loss of Cdk4 in hematopoietic cells does not alter response to allergen challenge. (A) Schematic cartoon of allergen challenge. Cdk4−/− and WT bm chimeric mice were sensitized and challenged with PBS or OVA. (B) Total cell counts and counts for macrophages, eosinophils, neutrophils and lymphocytes in bronchoalveolar lavage fluid are shown. N = 10/group. (C) Invasive measurement of airway reactivity to i.v. acetylcholine. Results are means ± SEM (n = 4 to 5). Data is expressed as dose response to Acetylcholine (µg/g body weight (BW)).
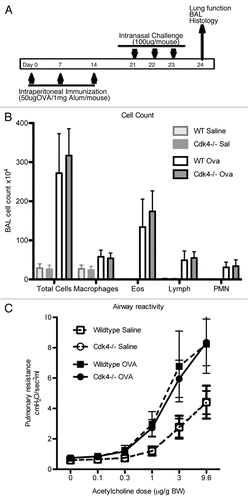
Figure 5 Loss of Cdk4 in hematopoietic cells does not alter response to allergen challenge. (D) Serum IgE content was measured by ELISA. (E) Cytokine measurements in bronchoalveolar lavage fluid. Values are means ± SEM for 10 mice per group (p < 0.05 compared with PBS sensitization and challenge). All mice are bm chimeras.
Acknowledgements
This work was supported by American Heart Association Grant-in-Aid, NIH HL083481, K24 HL086796 (L.M.S.) and HL18645 (J.H.).
References
- Besson A, Assoian RK, Roberts JM. Regulation of the cytoskeleton: An oncogenic function for CDK inhibitors?. Nat Rev Cancer 2004; 4:948 - 955
- Rane SG, Dubus P, Mettus RV, Galbreath EJ, Boden G, Reddy EP, et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in beta-islet cell hyperplasia. Nat Genet 1999; 22:44 - 52
- Sherr CJ, Roberts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev 2004; 18:2699 - 2711
- Tsutsui T, Hesabi B, Moons DS, Pandolfi PP, Hansel KS, Koff A, et al. Targeted disruption of CDK4 delays cell cycle entry with enhanced p27(Kip1) activity. Mol Cell Biol 1999; 19:7011 - 7019
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, et al. Mammalian cells cycle without the D-type cyclin-dependent kinases Cdk4 and Cdk6. Cell 2004; 118:493 - 504
- Sicinska E, Aifantis I, Le Cam L, Swat W, Borowski C, Yu Q, et al. Requirement for cyclin D3 in lymphocyte development and T cell leukemias. Cancer Cell 2003; 4:451 - 461
- Liu L, Schwartz B, Tsubota Y, Raines E, Kiyokawa H, Yonekawa K, et al. Cyclin-dependent kinase inhibitors block leukocyte adhesion and migration. J Immunol 2008; 180:1808 - 1817
- Harris NL, Watt V, Ronchese F, Le Gros G. Differential T cell function and fate in lymph node and nonlymphoid tissues. J Exp Med 2002; 195:317 - 326
- Wardlaw AJ, Guillen C, Morgan A. Mechanisms of T cell migration to the lung. Clin Exp Allergy 2005; 35:4 - 7
- Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, et al. A sensory neuronal ion channel essential for airway inflammation and hyperreactivity in asthma. Proc Natl Acad Sci USA 2009; 106:9099 - 9104
- Chen C, Huang X, Sheppard D. ADAM33 is not essential for growth and development and does not modulate allergic asthma in mice. Mol Cell Biol 2006; 26:6950 - 6956
- Suzuki R, Kuroda H, Komatsu H, Hosokawa Y, Kagami Y, Ogura M, et al. Selective usage of D-type cyclins in lymphoid malignancies. Leukemia 1999; 13:1335 - 1342
- Salomon DR, Mojcik CF, Chang AC, Wadsworth S, Adams DH, Coligan JE, et al. Constitutive activation of integrin alpha4beta1 defines a unique stage of human thymocyte development. J Exp Med 1994; 179:1573 - 1584
- Crisa L, Cirulli V, Ellisman MH, Ishii JK, Elices MJ, Salomon DR. Cell adhesion and migration are regulated at distinct stages of thymic T cell development: The roles of fibronectin, VLA4 and VLA5. J Exp Med 1996; 184:215 - 228
- Medoff BD, Thomas SY, Luster AD. T cell trafficking in allergic asthma: The ins and outs. Annu Rev Immunol 2008; 26:205 - 232