Abstract
Skin wound healing is a complex regenerative process involving various cell types. We recently investigated whether fetal microchimeric cells (FMCs) acquired during gestation contribute to maternal wound healing and used fetal microchimerism to investigate the recruitment of distant endothelial progenitor cells in skin wounds. Our study showed that fetal progenitor cells are recruited into maternal wounds and participate in inflammation and angiogenesis. These fetal cells might have beneficial effects in situations of maternal defective healing, and might also modify the adult maternal wound environment toward a scarless fetal-like wound healing.
Skin wound healing is a complex regenerative process involving various cell types and biological systems. It is schematically divided into three overlapping stages: inflammation, tissue synthesis and remodelling.Citation1 Conflicting data have been published regarding the participation of distant endothelial progenitor cells (EPCs) to the angiogenesis in the wound bed.Citation2-Citation4 These studies used chimeric mice obtained by transplantation of a tagged bone marrow from a transgenic mouse into a wild type (WT) recipient after a myeloablative treatment usually chemotherapy or irradiation. This conditioning may modify the natural course of the healing process. Natural chimerism occurs in parous and pregnant females as a result of the trafficking of fetal cells to the maternal circulation during pregnancy and their long-term persistence after delivery.Citation5-Citation8 Fetal microchimeric cells (FMCs) have been identified in larger numbers in pathological conditions, including cancer, inflammatory diseases or reparative processes as compared with normal tissues.Citation9-Citation15 This recruitment capacity associated with their described plasticityCitation7,Citation16 has suggested that the study of fetal cell microchimerism can inform on homing and engraftment of host stem cells in response to tissue injury. We recently investigated whether fetal microchimeric cells contributed to maternal wound healing and used this model to investigate the recruitment of distant EPCs in this process.Citation17 Tracking GFP+ fetal cells in WT females, we showed that FMCs were constantly present in high numbers in maternal wounds reaching hundreds per 104 maternal cells. Their numbers dramatically decreased to undetectable levels when the wound healed. Interestingly, in chronic wounds performed on Bleomycin-induced fibrotic skin, FMCs were still present at high levels on late time points after wounding as long as wounds were still open. FMCs were mainly CD45+ leukocytes at early stages and vWF+ endothelial cells at later stages. This followed the expected sequence of events during skin wound healing.Citation2,Citation3 In 2/11 mice, we identified vessels entirely constituted of fetal GFP+ endothelial cells and showed that they were branching and were connected to maternal circulation on adjacent serial sections. Besides we observed an amplification of circulating GFP+ cells mainly CD34+CD11b−VEGFR2− two days after skin wounding. These findings along with previous reports, including ours, converge toward the presence of fetal EPCs among the transferred FMCs.Citation11,Citation12,Citation18,Citation19 Two important questions that we addressed were as follows: were FMCs recruited in high numbers de novo or did randomly transferred FMCs proliferate in situ after tissue injury? Did fetal EPCs respond to specific chemotactic and angiogenic signals as their maternal counterparts? To answer these questions, we implanted Matrigel plugs spiked with recombinant VEGFa in the sub-cutis of pregnant mice. By detecting fetal GFP+ CD31+ endothelial cells in VEGFa-spiked Matrigel, we showed that fetal EPCs were recruited toward an acellular Matrigel plug in response to VEGFa signaling. Thus, FMCs that were detected in maternal skin wounds were, at least partly, recruited de novo in response to specific signals along with their maternal counterparts.Citation17
The transfer of wild type cells has been reported to improve diabetic chronic wounds in parabiotic mice.Citation20 It is therefore tempting to assume that normal FMCs might have beneficial effects in situations of maternal defective healing where these will display a selective advantage and possibly rescue the affected skin.Citation20,Citation21 Indeed, we have previously observed the transient healing of a recalcitrant leg ulcer in a sickle cell patient during pregnancy and hypothesized that it might be due to the recruitment of healthy fetal cells from her offspring.Citation22
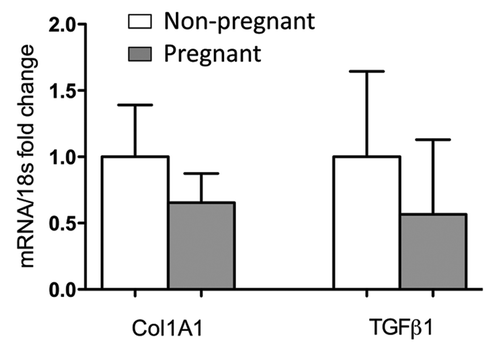
We next hypothesized that the presence of fetal cells recruited into normal wounds during pregnancy might modify the adult maternal wound environment toward a scarless fetal-like wound healing.Citation23 Although a difference in collagen deposition is hard to assess on histology, Collagen 1A1 and TGFβ transcripts were respectively decreased by 34% and 43% in wound beds of pregnant compared with non-pregnant mice (). This decrease did not reach statistical significance. Of course, this might be due to many accompanying physiological changes in pregnant mice. Nevertheless, this raises questions about the advantage of using fetal stem cells instead of adult derived stem cells in cell therapies for wound regeneration.
Figure 1.Collagen1A1 and TGFβ1 transcripts in skin wounds on pregnant and virgin mice. Surgical wounds (5 mm diameter) were performed on pregnant (E10) and virgin mice matched for age (n = 7 for each group). Seven days after wounding, wounds were harvested and mRNA extracted. Real-time PCR was conducted using SYBR®GREENPCR Master Mix (Applied Biosystems). mRNA values were normalized to the expression level of 18s RNA. Each sample was analyzed in duplicate. Histogram represents means +/− SEM.
In conclusion, the study of fetal microchimerism generates new concepts about the potential of fetal stem cells in therapy.
| Abbreviations: | ||
| WT | = | wild type |
| GFP | = | enhanced green fluorescent protein |
| vWF | = | von Willebrand factor |
References
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999; 341:738 - 46; http://dx.doi.org/10.1056/NEJM199909023411006; PMID: 10471461
- Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 1999; 85:221 - 8; PMID: 10436164
- Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004; 22:812 - 22; http://dx.doi.org/10.1634/stemcells.22-5-812; PMID: 15342945
- Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 2011; 117:5264 - 72; http://dx.doi.org/10.1182/blood-2011-01-330720; PMID: 21411758
- Bianchi DW, Zickwolf GK, Weil GJ, Sylvester S, DeMaria MA. Male fetal progenitor cells persist in maternal blood for as long as 27 years postpartum. Proc Natl Acad Sci U S A 1996; 93:705 - 8; http://dx.doi.org/10.1073/pnas.93.2.705; PMID: 8570620
- Khosrotehrani K, Johnson KL, Cha DH, Salomon RN, Bianchi DW. Transfer of fetal cells with multilineage potential to maternal tissue. JAMA 2004; 292:75 - 80; http://dx.doi.org/10.1001/jama.292.1.75; PMID: 15238593
- Khosrotehrani K, Bianchi DW. Multi-lineage potential of fetal cells in maternal tissue: a legacy in reverse. J Cell Sci 2005; 118:1559 - 63; http://dx.doi.org/10.1242/jcs.02332; PMID: 15811948
- O’Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, et al. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet 2004; 364:179 - 82; http://dx.doi.org/10.1016/S0140-6736(04)16631-2; PMID: 15246731
- Khosrotehrani K, Reyes RR, Johnson KL, Freeman RB, Salomon RN, Peter I, et al. Fetal cells participate over time in the response to specific types of murine maternal hepatic injury. Hum Reprod 2007; 22:654 - 61; http://dx.doi.org/10.1093/humrep/del426; PMID: 17074776
- Nelson JL. Microchimerism and human autoimmune diseases. Lupus 2002; 11:651 - 4; http://dx.doi.org/10.1191/0961203302lu271oa; PMID: 12413060
- Nguyen Huu S, Oster M, Uzan S, Chareyre F, Aractingi S, Khosrotehrani K. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci U S A 2007; 104:1871 - 6; http://dx.doi.org/10.1073/pnas.0606490104; PMID: 17267612
- Nguyen Huu S, Oster M, Avril MF, Boitier F, Mortier L, Richard MA, et al. Fetal microchimeric cells participate in tumour angiogenesis in melanomas occurring during pregnancy. Am J Pathol 2009; 174:630 - 7; http://dx.doi.org/10.2353/ajpath.2009.080566; PMID: 19147820
- Bou-Gharios G, Amin F, Hill P, Nakamura H, Maxwell P, Fisk NM. Microchimeric fetal cells are recruited to maternal kidney following injury and activate collagen type I transcription. Cells Tissues Organs 2011; 193:379 - 92; http://dx.doi.org/10.1159/000321172; PMID: 21150166
- Dubernard G, Aractingi S, Oster M, Rouzier R, Mathieu MC, Uzan S, et al. Breast cancer stroma frequently recruits fetal derived cells during pregnancy. Breast Cancer Res 2008; 10:R14; http://dx.doi.org/10.1186/bcr1860; PMID: 18271969
- Dubernard G, Oster M, Chareyre F, Antoine M, Rouzier R, Uzan S, et al. Increased fetal cell microchimerism in high grade breast carcinomas occurring during pregnancy. Int J Cancer 2009; 124:1054 - 9; http://dx.doi.org/10.1002/ijc.24036; PMID: 19065666
- Lee ES, Bou-Gharios G, Seppanen E, Khosrotehrani K, Fisk NM. Fetal stem cell microchimerism: natural-born healers or killers?. Mol Hum Reprod 2010; 16:869 - 78; http://dx.doi.org/10.1093/molehr/gaq067; PMID: 20663958
- Nassar D, Droitcourt C, Mathieu-d’Argent E, Kim MJ, Khosrotehrani K, Aractingi S. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J 2012; 26:149 - 57; http://dx.doi.org/10.1096/fj.11-180695; PMID: 21974929
- Parant O, Dubernard G, Challier JC, Oster M, Uzan S, Aractingi S, et al. CD34+ cells in maternal placental blood are mainly fetal in origin and express endothelial markers. Lab Invest 2009; 89:915 - 23; http://dx.doi.org/10.1038/labinvest.2009.55; PMID: 19488036
- Kara RJ, Bolli P, Karakikes I, Matsunaga I, Tripodi J, Tanweer O, et al. Fetal cells traffic to injured maternal myocardium and undergo cardiac differentiation. Circ Res 2012; 110:82 - 93; http://dx.doi.org/10.1161/CIRCRESAHA.111.249037; PMID: 22082491
- Pietramaggiori G, Scherer SS, Alperovich M, Chen B, Orgill DP, Wagers AJ. Improved cutaneous healing in diabetic mice exposed to healthy peripheral circulation. J Invest Dermatol 2009; 129:2265 - 74; http://dx.doi.org/10.1038/jid.2009.60; PMID: 19295612
- Khosrotehrani K, Leduc M, Bachy V, Nguyen Huu S, Oster M, Abbas A, et al. Pregnancy allows the transfer and differentiation of fetal lymphoid progenitors into functional T and B cells in mothers. J Immunol 2008; 180:889 - 97; PMID: 18178828
- Droitcourt C, Khosrotehrani K, Girot R, Aractingi S. Healing of sickle cell ulcers during pregnancy: a favourable effect of foetal cell transfer?. J Eur Acad Dermatol Venereol 2008; 22:1256 - 7; http://dx.doi.org/10.1111/j.1468-3083.2008.02602.x; PMID: 18429979
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008; 453:314 - 21; http://dx.doi.org/10.1038/nature07039; PMID: 18480812