Abstract
The NIMA paradox is the observation that in transplants of allogeneic kidneys or hematopoietic stem cells, siblings benefit from re-exposure to non-inherited maternal antigens (NIMA), whereas re-exposure to a transplant from mother herself, theoretically the ideal “NIMA” donor, does not yield clinical results superior to a father-donated allograft. Recent observations of bidirectional alloreactivity in kidney and cord blood transplantation offer a possible solution to this paradox. If correct, the proposed solution points the way to clinical applications of microchimerism in solid organ and hematopoetic transplants.
Introduction
The purpose of this brief review is to re-examine a major paradigm of transplant immunology in light of two recent findings: one in the field of cord blood (CB) transplantation for the treatment of acute leukemia in HLA-mismatched third party recipients, the other in the field of kidney transplantation using living related donor-recipient pairs. In doing so, we will revisit the “two-way” theory of transplantation tolerance proposed 20 years ago by Dr. Thomas Starzl, based on the finding of microchimerism in long-surviving human liver transplants.
Big Effects From Tiny Amounts of Maternal Cells: A Hidden Benefit of Cord Blood Transplantation
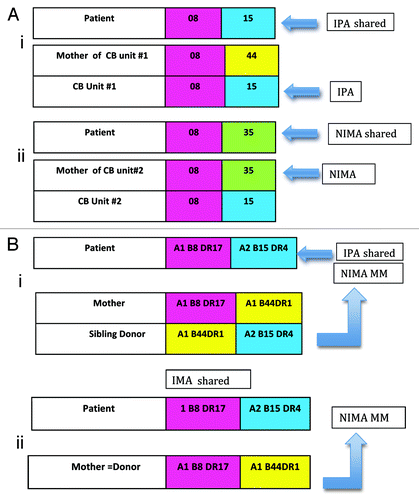
Cord blood transplantation was first performed in 1988 by Gluckman et al.,Citation1 as a therapy for Fanconi anemia. Subsequently, when cord blood transplantation was just beginning to be used as therapy for leukemia, oncologists and hematologists were delighted to tap the rich source of HSCs present in umbilical cord blood (CB), and did not mind the admixture of donor T lymphocytes.Citation2,Citation3 Indeed they regarded the neonatal T cell as fairly benign and therefore, unlike adult T cells, unlikely to cause graft vs. host disease (GVHD), a prediction that was borne out in clinical practice.Citation4 However, they were also transferring adult T cells of maternal origin—accounting presumably for 0.1–0.5% of the total T cells in cord blood.Citation5,Citation6 A typical CB transplant involves transfer of 3 × 107 nucleated cells per kg recipient body weight, and assuming that 1% are CD34+ HSC and 33% are T cells, this means as many as 5 × 104 maternal T cells/kg were being transferred with each CB treatment. Jon van Rood and collaborators Cladd Stevens and Andromachi Scaradavou hypothesized that these few maternal T cells, presumably memory cells, may be enriched for reactivity to the inherited paternal antigens (IPA) of the baby, and therefore could mediate potent graft-vs. leukemia effects if the leukemia patient happened to share this same IPA. A retrospective analysis of the New York Blood Center National Cord Blood program transplant database, in which both the mothers and the neonatal donors had been HLA-typed, was performed. As shown in , transplants could be assigned to an “IPA-shared” or “IPA non-shared” category on the basis of the HLA typings of the mother, the cord blood donor, and the leukemia patient—in the example shown, HLA-B15 was identified as an IPA in the CB donor since mother lacks this antigen, and this same HLA-B antigen was also present in the patient. The sharing of IPA between the CB donor and recipient was found to be advantageous to recipients with either acute myeloid leukemia (AML) or acute lymphoblastic leukemia (ALL), significantly limiting relapse rates in the first 3 y post-transplant, in comparison with patients where no IPA sharing was present.Citation7 The benefit of IPA sharing was achieved with only a slight increase in GVHD incidence (HR 1.4) that did not reach statistical significance, while the benefit in terms of reduced relapse risk was substantial and highly significant (HR = 0.35, p < 0.001).Citation7 All this with only the HLA mismatches between mother and CB donor analyzed—minor H IPA mismatches might play an even greater role, but typing for these is still in its infancy. The same investigators previously found that NIMA matching between the mother and leukemia patient promotes engraftment and increased survival rate in CB transplants for AML.Citation8 Unlike those findings, it is difficult to account for the benefit of IPA-sharing between the CB donor and eventual AML or ALL patient based on the effects of the transferred CB donor T cells alone. The only logical explanation for these remarkable retrospective data on IPA-shared CB transplants is that the rare maternal T or B cells co-transferred with the CB must mediate the beneficial effect of IPA sharing. However, proof of this concept will require direct isolation and functional analysis of the maternal lymphocytes involved. A recent report of transmaternal immunization of cord blood (fetal) T memory cells to HY minor H antigens, possibly from an older male sibling, is an exciting development that illustrates the complexity of the task ahead in this new area of T cell biology.Citation9
Figure 1. (A) Cord blood transplantation: the ipa and nima effects. (i) IPA effect. Anti-relapse benefit because the mother’s T cell would be sensitized to B*15 and the patient has B*15 (shared IPA). (ii) NIMA effect. Transplant related mortality reduced with CB#2 because the baby donor has tolerance to the non-inherited maternal HLA B*35 and the patient has B*35 (shared NIMA), presumably facilitating engraftment. (B) Renal transplantation: the nima paradox. (i) Results, 80% graft survival at 10 y; significantly (p<.006) different from 50% survival at 10 y for IMA-shared, NIPA-MM sibling donor transplant. (ii) Results, 50% graft survival at 10 y. Not significantly different from paternal donor transplant survival.
Big Effects from Kidney “Passenger” Leukocytes: The Discovery of Bidirectional Regulation in Living-Related Kidney Transplantation
A clinical study was begun in Madison in 2005, to determine (1) if immune regulation could be detected toward alloantigens of family members (mother, father, son, daughter and sibling) using a “bystander suppression” assay; and (2) if pre-transplant regulation status could be used as a tool to distinguish tolerance-prone from rejection-prone donor-recipient pairs within families. This trial was not motivated by a desire to solve the NIMA paradox—why kidney grafts from siblings mismatched for the NIMA-HLA haplotype do so much better than grafts from siblings mismatched for NIPA- HLA,Citation10 while grafts the mother herself do no better, or possibly worse,Citation11 than grafts from the father (for a more detailed illustration of the NIMA paradox in renal transplantation, please see ). Rather, it was motivated by a curious observation during routine post-transplant monitoring of HLA-identical sibling transplant recipients tested using a trans-vivo delayed type hypersensitivity (tvDTH) inhibition assay. We found that 12/12 of these patients exhibited bystander suppression—a > 50% inhibition of a third-party recall response to EBV or tetanus toxoid (TT) if those antigens were mixed with recipient PBMC and injected into a SCID mouse footpad along with a sonicate of cells from the HLA-identical donor.Citation12,Citation13 We wondered if the regulatory responses had been induced by exposure to sibling minor H antigens [miHA] during the course of transplantation, or if the miHA-specific Tregs were already present prior to transplant, induced by fetal-maternal cell exchange. We knew from our previous work on a patient with > 30 y of tolerance to a renal transplant from her HLA-identical sister, that CD8+CTLA4+Foxp3neg regulatory T cells specific for the donor's miHA HA-1 were present in her peripheral blood. These cells could suppress IFNγ production and DTH reactions mediated by HA-1-specific CD8 T effector/CTL also present in her blood; blockade of CTLA4, or neutralization of TGFβ and IL10, revealed the T effector responses. Most intriguing, CD8+ HA1-specific effector and regulatory T cells could be distinguished on the basis of binding to HA-1H peptide/HLA-A2 tetramers, the effector T cells staining brightly, regulatory cells dimly.Citation13 Both types of HA-1-specific T cells co-existed in peripheral blood with HA-1 microchimerism in T cells and dendritic cells. Since the miHA in question, HA-1, is normally expressed only by leukocytes, and not by kidney parenchymal cells,Citation14 T/DC microchimerism was the likely source of antigen sustaining the HA-1 specific T cells. Since HA-1 microchimerism could have derived from the sibling donor, or from maternal (NIMA) or fetal (she had a HA-1+ daughter prior to her renal failure) exposures prior to the transplant, we felt it was at least possible that HLA-identical sibs in general might be regulatory toward each other’s miHA.
For this reason we decided to test regulation status in seven patients who were about to receive a HLA-identical kidney transplant from a sibling, 1 d prior to transplant surgery. If no regulation was found, then the regulation seen in HLA-ID recipients after transplant must be the result of kidney transplantation. As a control for disease status, the healthy sibling donors were also tested for regulation toward the HLA-identical recipient’s antigens. Briefly, PBMC (recipient or donor) were obtained for use as responder cells in a trans-vivo delayed type hypersensitivity (tvDTH) assayCitation15; a portion of these cells were sonicated and prepared for use as soluble antigen to be injected, along with PBMC in the footpad of a CB.17 SCID mouse. As a positive control, tetanus toxoid (TT) was co-injected with PBMC to obtain a strong tvDTH swelling response. Regulation could be measured on a scale of 0–100%, by the relative decrease in caused by the admixture of soluble antigen to the PBMC and TT in the footpad challenge and 24hr swelling assay. If T regs specific for a donor or recipient minor H peptide were present in either recipient or donor PBMC, recognition of peptide(s) in the soluble antigen preparation will cause bystander suppression of the effector T cells responding to TT. The actual mechanism of suppression—TGFβ, IL10, adenosine or IL35—may vary from patient to patient, but the readout is the same. The results of pre-transplant analysis of antigen-specific regulatory T cells using tvDTH were striking—not only did the patient with end-stage-renal disease have regulatory T cells specific to the miHA of his or her HLA-identical sibling (inhibition ≥ 50%), but the donor’s PBMC also contained regulatory T cells specific to the miHA of the recipient. Autologous sonicates, or sonicates from an identical twin,Citation16 failed to stimulate bystander suppression, ruling out a non-specific effect of sonicates. The sum of inhibition in each direction or “combined regulation score” (donor anti-recipient plus recipient anti-donor) were in excess of 100% for all HLA-ID sib pairs tested. Whether the presence of these miHA-specific Tregs prior to transplantation were the result of pre-existing maternal or fetal microchimerism was not determined.Citation17 In a non-depleting (induction-free) protocol, using only short-term corticosteroids and maintenance immunosuppression with mycophenolate and calcineurin inhibitors (CNI), the clinical results for these patients were all outstanding in terms of renal function at 3 y, and lack of rejection, as expected for kidney transplants with a predicted half-life of > 23 y. The success of the HLA-identical sibling kidney is classically attributed simply to the absence of HLA differences, rendering such grafts safe from the ravages of anti-HLA, donor-specific T cells and antibodies (DSA). Was pre-transplant bidirectional regulation, described here for first time, a contributing factor to the success of the HLA-identical sibling renal transplant? Since regulation was uniformly bi-directional, and the outcomes uniformly excellent, there was no way of assessing the role of regulation originating from either the donor and recipient side alone.
Fortunately, we had also included 18 HLA-haplotype mismatched, as well as 4 living unrelated donor-recipient pairs in the study. The living-unrelated pairs did not exhibit pre-transplant regulation, indicating that such regulation could be a specific product of maternal-fetal exchange and not a non-specific phenomenon. We found variable levels of regulation in the haplotype mismatched pairs depending upon family relationships. The one consistent vector for regulatory responses in adult HLA haploidentical pairs was that of offsprings’ PBMC toward their mother’s antigens. This finding of uniform NIMA-specific regulation mediated via the “indirect” pathway (allo-antigens presented by recipient APCs) was somewhat unexpected, because previous attempts to demonstrate NIMA tolerance at the T cell level in adults using in vitro MLR-based techniques (measuring only direct alloresponsivess) had failed.Citation18-Citation20 The results illustrate the importance of monitoring indirect alloreactivity by T cells in the assessment of natural alloreactivity and tolerance.Citation16
In contrast, the response of the mothers to the inherited paternal antigens (IPA) expressed by their son or daughter was strikingly different from the anti-NIMA responses of their offspring. The healthy mothers either did not suppress at all (0%), or regulated weakly (30%) the response to the IPA expressed by the offsprings. Only two mothers, both type 1 diabetics with ESRD, regulated strongly (> 60%) to the IPAs of their daughters, their prospective kidney donors. Because the healthy donors were both highly regulatory toward their respective NIMAs (both > 60%), there was a high combined inhibition score for these 2 mother-daughter pairs. Other HLA haplotype mismatched pairs, including siblings, and father—offspring pairs, were a mixed bag—some pairs exhibited bi-directional regulation, others not.
All recipients of HLA-haplo MM living related, and living unrelated kidney transplants were treated with CAMPATH-1H(anti-CD52 humanized mouse monoclonal Ab), a strongly depleting induction agent. The outcomes of the renal transplants in the 18 HLA haplo MM donor-recipient pairs revealed quite striking differences depending on pre-transplant regulation scores. Recipient-side regulation alone did not predict for excellent renal function at 3 y; however, the association of a combined regulation score (donor plus recipient) with 3 y renal function was significant (p < 0.01, p < 0.05 for serum creatinine < 1.5 mg/dl, creatinine clearance > 50ml/min/1.73m2, respectively). Furthermore, when the HLA haploMM D-R pairs were subdivided into bidirectional vs. unidirectional/no regulation groups (see ref. Citation17) significant differences in renal function emerged as early as 6 mo post-transplant, growing wider over time. By 3 y, 4/9 transplants in the uni/non regulator group had been lost, 7/9 had had at least one rejection episode, and 5/9 had developed anti-HLA class II donor-specific antibody (DSA). In contrast, 1/9 in the bidirectional group had had a mild humoral rejection episode on d.8, associated with a class I-specific DSA. This patient, one of the two mothers who received a transplant from their daughter, had a very high combined regulation score pre-transplant (167%); her rejection was reversed and she went on to have an uneventful course, with a sCr of 1.1 mg/dl at 3 y. The rapid tempo of onset of a first rejection episodes in a bidirectional regulator was highly reminiscent of the situation of the NIMA vs. NIPA-MM siblings—enhanced long-term graft survival in the NIMA-sharing sibs was associated with a rapid onset of first rejection (within 2 weeks), while NIPA-sharing sibs has much delayed first rejection, but overall lower 10 y graft survival.Citation10 Overall, results in the subgroup of bidirectional regulators were comparable to that in the HLA-identical sibling transplants, and parallel to the results of NIMA-MM siblings reported previouslyCitation10.
One factor that may have accentuated the differences between poor and excellent transplant outcomes was the immunosuppressive protocol. All of the HLA haploMM patients received Campath-1H as an induction agent at the time of transplant, and some received additional treatment with anti-CD20 (Rituximab); this highly depleting regimen is now known to increase complications due to antibody and non-classical forms of rejection.Citation21 It is also unclear how many donor T cells are present in kidney allografts, and of these how many could survive the depletion regimen to exert such a profound influence on outcome. Even so, at the very least these results challenge the classical paradigm of a uni-directional transplantation, host vs. graft model of transplantation, arguing for a new, bidirectional paradigm. If verified in a larger series of patients, the bidirectional paradigm will not only offer a resolution of the NIMA paradox, but also points the way to strategies to exploit natural allotolerance/microchimerism for induction of mixed chimerism/ tolerance in the clinical setting. Indeed the one patient subgroup where stable mixed chimerism along with a kidney transplant acceptance has been achieved is the HLA-identical sibling.Citation22 Attempts to induced stable mixed chimerism in 1 haplotype-MM kidney transplants have been thus far unsuccessful.Citation23 Considering the excellent results observed in 1 haplotype-MM kidney transplants with pre-transplant bi-direction regulation, perhaps this is the subgroup that will provide the first instances of mixed chimerism tolerance.
Mechanisms: The Immune System of the Transplanted Organ as Key to Tolerance
The field of transplant immunology began with the observation by Ray Owen of mixed chimerism—a 50:50 mixture of red blood cell types in dizgotic cattle twins—which he correctly inferred was the result of a bidirectional, acquired immunologic tolerance resulting from placental fusion and parabiosis of the twins in utero.Citation24 Medawar was doing his own experiments on cattle twins around the same time. He had found that non-primarily vascularized skin allografts evoked a massive rejection response after an initial healing-on period.Citation25 Unaware of Owen’s work, he found that dizygotic cattle twins were reciprocally tolerant to each others’ skin, while rejecting third party skin grafts normally.Citation26 Billingham, Brent and MedawarCitation27 later reproduced the effect of neonatal tolerance in a mouse skin allograft model by transferring donor cells shortly after the birth of the eventual graft recipient. As discussed in a previous issue of Chimerism,Citation28 Medawar was convinced that between the time of introduction of allogeneic cells into the neonate, and the time of skin transplant in the adult “antigens must continue to be present, even though in quantities below the threshold of direct estimation, if a fully non-reactive state is to be maintained”Citation29. This conviction was doubtless born of his and Owen’s early observation of the bi-directional nature of tolerance in the cattle twins. Starzl calls this the “lost chord” in transplantation immunology, since between the experiments of Simonsen in the late 1950sCitation30 and the discovery of systemic male microchimerism in female recipients of male liver transplantsCitation31,Citation32 a disease-pathogen defense paradigm had dominated transplantation as well as the wider field of immunology. This may account for the intense controversy in which opponents of Starzl’s interpretation of the liver transplant data argued that microchimerism was not a fundamental principle of tolerance but merely a side effect.Citation33 Recently, a different paradigm of immunobiology, that innate and adaptive immunity are the products of co-evolution with viral, bacterial, fungal and helminth pathogens, optimizing survival of host and parasite, has emerged.Citation28,Citation34 The evidence for a big impact of a few maternal T cells in CB transplantation for acute leukemia, and data suggesting a big impact of the passenger leukocytes, including T cells, in kidney allograft survival in a depletional protocol, reinforce this paradigm shift, but begs the question of mechanism.
What mechanism(s) could account for the bi-directional nature of transplant tolerance? Here are the major components that any theory of mechanism ought to consider: (1) capacity for apoptosis, anergy and regulation, (2) route of access of donor passenger cells to host, (3) immune status of organ/tissue and (4) pathway of allorecognition.
Capacity for Apoptosis, Anergy and Regulation
The rodent organ transplant literature offers stark contrasts in the tolerogenic potential of different organ transplants. For example, both liver and kidney transplants in mice and rats may be spontaneously accepted, whereas skin grafts, orthoptopic lung are heterotopic heart allografts are rejected in the same strain combinations. The mechanism of spontaneous tolerance of liver transplants relies heavily upon apoptosis in alloreactive T cells, due to rapid migration of passenger leukocytes to the spleen and lymph node where a bidirectional, activation-induced cell death occurs.Citation35,Citation36 These results are consistent with the mutual clonal exhaustion/deletion model of bidirectional tolerance proposed by Starzl based on the human liver transplant experience.Citation37 In contrast, spontaneous mouse kidney allograft tolerance appears to involve little in the way of apoptotic cell death; rather the CD4+CD25+ T regulatory cells of the host (and donor?) appear to be critical, and there is a distinctive pattern of Treg and B cell infiltration in the cortex that occurs within 72 h of graft placement.Citation38,Citation39 Similar patterns of Treg-enriched organized lymphoid structures (TOLS) have been described in accepted monkeyCitation40-Citation42 and humanCitation43 kidney allografts, while elevated naive B cell counts in the periphery and CD20 mRNA in urine samples of tolerant patients have recently been described.Citation44,Citation45 In addition, tolerance to a kidney transplant also involves anergy. The first demonstration of a role of anergy in human tolerance was associated with microchimerism in a case of metastable tolerance to a maternal kidney transplant; this anergy was due to the action of NIMA-HLA+ cells, including donor-derived microchimeric cells.Citation46
In murine heterotopic heart transplants, all three mechanisms—anergy, apoptosis and regulation—appear to be required for tolerance induction by donor-specific transfusion or DST, along with costimulation blockade with anti-CD40L antibody. The specific sequence is important—DST+ anti-CD40L treatment 7 d prior to transplant induces proliferation of donor antigen-specific (TcR transgenic) T cells that become regulatory (Foxp3+) cells. The same transgenic T cells introduced at the time of transplantation failed to proliferate in the transplant recipient (suggesting anergy) and many underwent apoptosis.Citation47 Bidirectional alloregulation due to pre-existing microchimerism-induced Tregs might be considered analogous to the situation in the DST+ anti-CD40L treated hosts—the abundance of regulation at the graft site may predispose to anergy and apoptosis in T cells specific each other’s antigens. If indeed the NIMA paradox can be replicated in mouse and monkey transplant models, much research will be required to sort out the relative contributions of regulation, anergy and apoptosis on the donor or recipient side, as well as the contribution of regulatory antigen-presenting cells of donor and recipient origin.
Route of Access of Donor Passenger Cells to the Host
The issue of donor passenger cell access to the host, and host-to-graft access in the immediate aftermath of the transplant will likely be a critical determinant in the mechanism of bidirectional tolerance. Although the first graft type to demonstrate acquired neonatal tolerance, the non-primarily vascularized skin allograft in adult mice is resistant to tolerization by the same methods of co-stimulation blockade that work in heart and lung allografts. Unlike organ transplants, conventional skin allografts are not vascularized at the time of their placement. Consequently, passenger leukocytes leave the graft exclusively through lymphatic vessels and infiltrate preferentially host’s draining lymph nodes where they elicit potent direct and indirect inflammatory responses. On the other hand, passenger leukocytes from heart and kidney allografts are more likely to migrate out of the graft via blood vessels and traffic through the recipient’s lymphoid and non-lymphoid tissues immediately after transplantation. At the same time, while tolerance to solid organ allografts is ready achievable in rodents via multiple protocols including DST + costimulation blockade, these treatments are unsuccssful with skin allografts.
However, we recently observed that when the skin allograft is transplanted as a vascularized pedicle, T cell costimulation blockade works as well to prolong graft survival as it does in heart transplantation (Kant C. et al., manuscript in submission). The immediate spreading of donor T cells to host lymphoid and non-lymphoid tissues and the reduced inflammation in the setting of a vascularized transplant may also cause a shift in host indirect response toward immunogenic, but hematopoetic-restricted minor antigens, such as HA-1 in humans or H-60 in mice, causing an immundominant response less likely to harm the graft parenchyma.Citation48 Alternatively, this shift in immunodominance could promote the indirect activation of Tregs directed to donor MHC class I and minor antigens.
Immune Status of Organ/Tissue
The concept of “tissue-appropriate” immune function has been recently introduced by Matzinger and KamalaCitation49 to account for certain regulatory phenomena seen in the course of immunity to pathogens. Tissue-based class control posits that the type of effector response (Th1, Th2, Th3, Th17, etc.) is not primarily controlled by the pathogen, but by the tissue where the response to the pathogen occurs. Thus for example, the T helper response to a gut microbe will be Th3-biased, due to the requirement for TGFβ for IgA production. This Th3 response will strongly suppress Th1 cells, and thus appear to be regulatory; but it is simply the tissue-appropriate response in the gut environment. This concept is highly reminiscent of ocular immune privilege and the anterior chamber associated immune deviation (ACAID) phenomenon, which places tremendous importance on the role of tissue APC in the control of immune responses that occur in the eye.Citation50 Thus, corneal transplants can be done with only short-term, topical immunosuppression, partly due to anatomical restrictions of blood and lymph supply, but partly due to tolerogenic effects of MHC class IIneg corneal DC.Citation51 This is associated with the lack of inflammatory CD4+ T cell direct alloresponse. On the other hand, indirect CD4+ T cell responses are regularly detected in mice recipients of fully allogeneic corneas. However, this response is unconventional in that it is directed exclusively to minor antigens and it fails to ensure the differentiation of CD8+ cytotoxic T cells. Consequently, in the B6 to BALB/c model, 50% of cornal allografts are spontaneously accepted while the remainder grafts undergo a slow process of rejection. Interestingly, when B6 corneal grafts are transplanted on an inflammatory eye bed (high-risk setting involving 15% of patients), these transplants elicit vigorous Th1 CD4+ direct alloresponses and undergo rapid and acute rejection.Citation52
In the case of cardiac transplants, the aforementioned DST+ anti-CD40L is effective in inducing allotolerance in wild-type mice, but not in CXC3R1 “null” mice naturally deficient in tissue-resident DC and macrophages.Citation53 Depletion of heart-resident tissue macrophages by peritransplant antibody treatment led to accelerated cardiac allograft vasculopathy in rats,Citation54 a phenomenon also seen in combined cardiac -bone marrow, but not in combined cardiac-liver transplantation, where vascular architecture and tissue resident macrophages were preserved long after transplant surgery.Citation55 Sustaining “tissue-appropriate” immunity immediately after transplantation is likely critical for long-term tolerance with excellent graft function. A critical role is played by tissue-resident DCs and macrophages, the latter being possibly identical to regulatory macrophages or “Mregs,”Citation56 a donor cell-based therapy currently being deployed in the service of pre- and post-transplant immune suppression in the EU-sponsored One Study (www.onestudy.org).
The importance of tissue-appropriate immunity is that it may manifest as regulation of Th1 and NK responses occurring in the immediate aftermath of transplant surgery. The new appreciation of the critical role of innate immunity and damage-associated molecular patterns (DAMPs) in the early post-transplant periodCitation57 means that, to be truly effective in preventing chronic rejection, transplant tolerance may require a rapid local immune-regulatory response to control ischemia-reperfusion injury in order to re-establish tissue homeostasis and avoid delayed graft function. Here the role of Tregs transplanted with the organ may be critical in enforcing a regulation response to the influx of recipient cells and antigens into the graft. The classical study of Graca et al.Citation58 showing that tissue resident Tregs in tolerated skin grafts were able to prolong survival of retransplanted allografts onto naïve hosts has recently been replicated in a model of orthotopic lung transplantation in mice.Citation59 The capacity to harbor T reg-enriched organized lymphoid structures (TOLS) in kidney allografts cited previouslyCitation39 may be shared with other epithelial tissues such as skin and lung, but not heart.Citation59 Whether the same principle of Treg-passenger cells applies to bidirectional regulation in living related kidney transplants remains to be proven.
Pathway of Allorecognition
Two major pathways of allorecognition that may contribute to bidirectional regulation have been termed direct and indirect, based on the realization that in addition to the high frequency of T cells recognizing foreign MHC/peptide complexes on the surface of an allogeneic antigen presenting cell (APC), it is possible for T cells of normal (low) frequency to recognize allopeptides derived from the same foreign MHC molecules on the surface of autologous APC, as complexes with self MHC molecules.Citation60,Citation61 The critical importance of the latter pathway in NIMA-induced regulation and subsequent tolerance to a heart transplant, in contrast to relative sparing of the direct pathway by maternal exposure, was recently described in a transgenic mouse model.Citation62 In this study, it was demonstrated that tolerance to allogeneic heart transplants could be transferred via adoptive transfer of recipients with NIMA-specific CD4+FoxP3+ Tregs presumably activated in an indirect fashion. These data corroborate years of study in humans showing lack of a NIMA effect on cytolytic T lymphocyte and lymphoproliferative responses in mixed lymphocyte reactions (MLR),Citation18,Citation20,Citation63 and the recent finding of a powerful tolerogenic effect on indirect pathway responses to NIMA as measured by linked suppression in the trans-vivo DTH assay.Citation17 The profound anergy of a direct pathway response to NIMA in a tolerant recipient of a maternal renal transplant provided the first solid evidence for bi-directional alloreactivity and microchimerism influencing T cell function.Citation46 However, it should be noted that this graft was eventually lost, just as most maternal kidney allografts are eventually lost due to rejection, apparently at a faster rate than any other HLA-haploMM from a living relative.Citation64 We favor the concept that an anti-IPA response from the maternal (donor) side is responsible for undermining tolerance to NIMA in the recipient, while mutual regulation between NIMA HLA-haploMM siblings reinforces tolerance, and we have offered this explanation as a solution to the NIMA paradox.Citation17 However, further work is needed to dissect the roles of direct vs. indirect pathway during loss of tolerance to a maternal allograft,Citation65,Citation66 and to determine whether maternal anti-IPA specific T cells are involved. It will also be interesting to explore clinical situations where maternal grafts are favored over paternal ones simply because end-stage organ failure may actually be caused by an anti-IPA response of maternally-derived microchimeric cells, as has recently been suggested for biliary atresia and its correction by live-donor liver transplantation.Citation67
Conclusion
This brief review has encompassed the NIMA paradox, the possible benefits of “passenger” anti-IPA maternal T cells in CB transplantation, and their apparent detrimental effects in live-related kidney transplantation. The existence of pre-transplant bi-directional regulation and its association with excellent transplant outcome in LR kidney transplantation raises many new questions concerning microchimerism-induced regulation and the role of the organ-specific immune system transplanted along with the graft. Mouse, rat and monkey models are needed to resolve the many mechanistic questions that remain. One potential implication of the finding of bidirectional vs. unidirectional regulation in HLA-haplotype MM transplants is that opportunities for induction of mixed chimerism may greatly improve in donor-recipient pairs with bidirectional regulation, and be disadvantaged in pairs with uni-directional or non-regulation, or in cases of direct pathway sensitization.Citation68 There may also be opportunities to infuse grafts with the proper dose of donor DCregs or Mregs or donor anti-recipient Tregs, in addition to recipient Treg treatments, as we enter the era of cell-based therapy as an alternative to pharmacologic immune suppression in clinical transplantation.
| Abbreviations: | ||
| CB | = | cord blood |
| DST | = | donor-specific transfusion |
| HLA-haplo MM | = | mismatched for 1 HLA haplotype (matched for the other) |
| HLA-ID | = | HLA-identical, matched for 2/2 parental HLA haplotypes |
| HSCs | = | hematopoietic stem cells |
| IPA | = | inherited paternal antigen |
| LR | = | living related |
| LUR | = | living unrelated |
| NIMA | = | non-inherited maternal antigen |
Reference List
- Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med 1989; 321:1174 - 8; http://dx.doi.org/10.1056/NEJM198910263211707; PMID: 2571931
- Broxmeyer HE, Hangoc G, Cooper S, Ribeiro RC, Graves V, Yoder M, et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc Natl Acad Sci U S A 1992; 89:4109 - 13; http://dx.doi.org/10.1073/pnas.89.9.4109; PMID: 1373894
- Wagner JE, Broxmeyer HE, Cooper S. Umbilical cord and placental blood hematopoietic stem cells: collection, cryopreservation, and storage. J Hematother 1992; 1:167 - 73; http://dx.doi.org/10.1089/scd.1.1992.1.167; PMID: 1365024
- Ballen K, Broxmeyer HE, McCullough J, Piaciabello W, Rebulla P, Verfaillie CM, et al. Current status of cord blood banking and transplantation in the United States and Europe. Biol Blood Marrow Transplant 2001; 7:635 - 45; http://dx.doi.org/10.1053/bbmt.2001.v7.pm11787526; PMID: 11787526
- Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, et al. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science 2008; 322:1562 - 5; http://dx.doi.org/10.1126/science.1164511; PMID: 19056990
- Hall JM, Lingenfelter P, Adams SL, Lasser D, Hansen JA, Bean MA. Detection of maternal cells in human umbilical cord blood using fluorescence in situ hybridization. Blood 1995; 86:2829 - 32; PMID: 7545474
- van Rood JJ, Scaradavou A, Stevens CE. Indirect evidence that maternal microchimerism in cord blood mediates a graft-versus-leukemia effect in cord blood transplantation. Proc Natl Acad Sci U S A 2012; 109:2509 - 14; http://dx.doi.org/10.1073/pnas.1119541109; PMID: 22232664
- van Rood JJ, Stevens CE, Smits J, Carrier C, Carpenter C, Scaradavou A. Reexposure of cord blood to noninherited maternal HLA antigens improves transplant outcome in hematological malignancies. Proc Natl Acad Sci U S A 2009; 106:19952 - 7; PMID: 19901324
- Dierselhuis MP, Blokland EC, Pool J, Schrama E, Scherjon SA, Goulmy E. Transmaternal cell flow leads to antigen-experienced cord blood. Blood 2012; 120:505 - 10; http://dx.doi.org/10.1182/blood-2012-02-410571; PMID: 22627770
- Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med 1998; 339:1657 - 64; http://dx.doi.org/10.1056/NEJM199812033392302; PMID: 9834302
- Opelz G. Study ftCT. Analysis of the 'NIMA effect' in renal transplantation. In: Terasaki PI, editor. Clinical Transplants 1990. 6 ed. Los Angeles, CA: UCLA Tissue Typing Laboratory; 1990. p. 63-7.
- Rodriguez DS, Jankowska-Gan E, Haynes LD, Leverson G, Munoz A, Heisey D, et al. Immune regulation and graft survival in kidney transplant recipients are both enhanced by human leukocyte antigen matching. Am J Transplant 2004; 4:537 - 43; http://dx.doi.org/10.1111/j.1600-6143.2004.00385.x; PMID: 15023145
- Cai J, Lee J, Jankowska-Gan E, Derks R, Pool J, Mutis T, et al. Minor H antigen HA-1-specific regulator and effector CD8+ T cells, and HA-1 microchimerism, in allograft tolerance. J Exp Med 2004; 199:1017 - 23; http://dx.doi.org/10.1084/jem.20031012; PMID: 15067036
- Wilke M, Dolstra H, Maas F, Pool J, Brouwer R, Falkenburg JH, et al. Quantification of the HA-1 gene product at the RNA level; relevance for immunotherapy of hematological malignancies. Hematol J 2003; 4:315 - 20; http://dx.doi.org/10.1038/sj.thj.6200318; PMID: 14502255
- Carrodeguas L, Orosz CG, Waldman WJ, Sedmak DD, Adams PW, VanBuskirk AM. Trans vivo analysis of human delayed-type hypersensitivity reactivity. Hum Immunol 1999; 60:640 - 51; http://dx.doi.org/10.1016/S0198-8859(99)00002-6; PMID: 10439310
- Haynes LD, Jankowska-Gan E, Sheka A, Keller MR, Hernandez-Fuentes MP, Lechler RI, et al. Donor-Specific Indirect Pathway Analysis Reveals a B-Cell-Independent Signature which Reflects Outcomes in Kidney Transplant Recipients. Am J Transplant 2012; 12:640 - 8; PMID: 22151236
- Jankowska-Gan E, Sheka A, Sollinger HW, Pirsch JD, Hofmann RM, Haynes LD, et al. Pretransplant immune regulation predicts allograft outcome: bidirectional regulation correlates with excellent renal transplant function in living-related donor-recipient pairs. Transplantation 2012; 93:283 - 90; http://dx.doi.org/10.1097/TP.0b013e31823e46a0; PMID: 22186938
- van den Boogaardt DE, van Miert PP, Koekkoek KM, de Vaal YJ, van Rood JJ, Claas FH, et al. No in vitro evidence for a decreased alloreactivity toward noninherited maternal HLA antigens in healthy individuals. Hum Immunol 2005; 66:1203 - 12; http://dx.doi.org/10.1016/j.humimm.2005.12.002; PMID: 16690407
- Roelen DL, van Bree FP, van Beelen E, van Rood JJ, Claas FH. No evidence of an influence of the noninherited maternal HLA antigens on the alloreactive T cell repertoire in healthy individuals. Transplantation 1995; 59:1728 - 33; http://dx.doi.org/10.1097/00007890-199506270-00015; PMID: 7541578
- Hadley GA, Phelan DL, Duffy BF, Mohanakumar T. Lack of T-cell tolerance of noninherited maternal HLA antigens in normal humans. Hum Immunol 1990; 28:373 - 81; http://dx.doi.org/10.1016/0198-8859(90)90032-K; PMID: 2391252
- Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, et al. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation 2003; 76:120 - 9; http://dx.doi.org/10.1097/01.TP.0000071362.99021.D9; PMID: 12865797
- Scandling JD, Busque S, Shizuru JA, Engleman EG, Strober S. Induced immune tolerance for kidney transplantation. N Engl J Med 2011; 365:1359 - 60; http://dx.doi.org/10.1056/NEJMc1107841; PMID: 21991976
- Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 2008; 358:353 - 61; http://dx.doi.org/10.1056/NEJMoa071074; PMID: 18216355
- Owen RD. Immunogenetic consequences of vascular anastomoses between bovine twins. Science 1945; 102:400 - 1; http://dx.doi.org/10.1126/science.102.2651.400; PMID: 17755278
- Medawar PB. Immunity to homologous grafted skin; the relationship between the antigens of blood and skin. Br J Exp Pathol 1946; 27:15 - 24; PMID: 20989196
- Anderson D, Billingham RE, Lampkin GH, Medawar P. The use of skin grafting to distinquish between monozygotic and dizygotic twins in cattle. Heredity 1951; 5:379 - 97; http://dx.doi.org/10.1038/hdy.1951.38
- Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature 1953; 172:603 - 6; http://dx.doi.org/10.1038/172603a0; PMID: 13099277
- Davies AJ. Immigration control in the vertebrate body with special reference to chimerism. Chimerism 2012; 3:1 - 8; http://dx.doi.org/10.4161/chim.20113; PMID: 22690266
- Medawar P. Immunological Tolerance. Nobel Lectures, Physiology or Medicine 1942-1962. Amsterdam: Elsevier; 1964.
- Simonsen M. The impact on the developing embryo and newborn animal of adult homologous cells. Acta Pathol Microbiol Scand 1957; 40:480 - 500; PMID: 13457893
- Starzl TE, Demetris AJ, Trucco M, Ramos H, Zeevi A, Rudert WA, et al. Systemic chimerism in human female recipients of male livers. Lancet 1992; 340:876 - 7; http://dx.doi.org/10.1016/0140-6736(92)93286-V; PMID: 1357298
- Starzl TE, Demetris AJ, Trucco M, Murase N, Ricordi C, Ildstad S, et al. Cell migration and chimerism after whole-organ transplantation: the basis of graft acceptance. Hepatology 1993; 17:1127 - 52; http://dx.doi.org/10.1002/hep.1840170629; PMID: 8514264
- Wood KJ, Sachs DH. Chimerism and transplantation tolerance: cause and effect. Immunol Today 1996; 17:584 - 7, discussion 588; http://dx.doi.org/10.1016/S0167-5699(96)10069-4; PMID: 8991291
- Medzhitov R, Schneider DS, Soares MP. Disease tolerance as a defense strategy. Science 2012; 335:936 - 41; http://dx.doi.org/10.1126/science.1214935; PMID: 22363001
- Bishop GA, Sun J, DeCruz DJ, Rokahr KL, Sedgwick JD, Sheil AG, et al. Tolerance to rat liver allografts. III. Donor cell migration and tolerance-associated cytokine production in peripheral lymphoid tissues. J Immunol 1996; 156:4925 - 31; PMID: 8648143
- Qian S, Demetris AJ, Murase N, Rao AS, Fung JJ, Starzl TE. Murine liver allograft transplantation: tolerance and donor cell chimerism. Hepatology 1994; 19:916 - 24; http://dx.doi.org/10.1002/hep.1840190418; PMID: 8138266
- Starzl TE, Zinkernagel RM. Antigen localization and migration in immunity and tolerance. N Engl J Med 1998; 339:1905 - 13; http://dx.doi.org/10.1056/NEJM199812243392607; PMID: 9862947
- Wang C, Cordoba S, Hu M, Bertolino P, Bowen DG, Sharland AF, et al. Spontaneous acceptance of mouse kidney allografts is associated with increased Foxp3 expression and differences in the B and T cell compartments. Transpl Immunol 2011; 24:149 - 56; http://dx.doi.org/10.1016/j.trim.2010.12.004; PMID: 21199671
- Miyajima M, Chase CM, Alessandrini A, Farkash EA, Della Pelle P, Benichou G, et al. Early acceptance of renal allografts in mice is dependent on foxp3(+) cells. Am J Pathol 2011; 178:1635 - 45; http://dx.doi.org/10.1016/j.ajpath.2010.12.024; PMID: 21435448
- Torrealba JR, Katayama M, Fechner JH Jr., Jankowska-Gan E, Kusaka S, Xu Q, et al. Metastable tolerance to rhesus monkey renal transplants is correlated with allograft TGF-beta 1+CD4+ T regulatory cell infiltrates. J Immunol 2004; 172:5753 - 64; PMID: 15100322
- Thomas JM, Carver M, Cunningham P, Park K, Gonder J, Thomas F. Promotion of incompatible allograft acceptance in rhesus monkeys given posttransplant antithymocyte globulin and donor bone marrow. I. In vivo parameters and immunohistologic evidence suggesting microchimerism. Transplantation 1987; 43:332 - 8; http://dx.doi.org/10.1097/00007890-198703000-00002; PMID: 3103273
- Haanstra KG, Sick EA, Ringers J, Wubben JA, Kuhn EM, Boon L, et al. Costimulation blockade followed by a 12-week period of cyclosporine A facilitates prolonged drug-free survival of rhesus monkey kidney allografts. Transplantation 2005; 79:1623 - 6; http://dx.doi.org/10.1097/01.TP.0000158426.64631.ED; PMID: 15940054
- Xu Q, Lee J, Jankowska-Gan E, Schultz J, Roenneburg DA, Haynes LD, et al. Human CD4+CD25low adaptive T regulatory cells suppress delayed-type hypersensitivity during transplant tolerance. J Immunol 2007; 178:3983 - 95; PMID: 17339499
- Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, et al, Immune Tolerance Network ST507 Study Group. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 2010; 120:1836 - 47; http://dx.doi.org/10.1172/JCI39933; PMID: 20501946
- Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, et al. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 2010; 120:1848 - 61; http://dx.doi.org/10.1172/JCI39922; PMID: 20501943
- Burlingham WJ, Grailer AP, Fechner JH Jr., Kusaka S, Trucco M, Kocova M, et al. Microchimerism linked to cytotoxic T lymphocyte functional unresponsiveness (clonal anergy) in a tolerant renal transplant recipient. Transplantation 1995; 59:1147 - 55; PMID: 7732562
- Burrell BE, Bromberg JS. Fates of CD4+ T cells in a tolerant environment depend on timing and place of antigen exposure. Am J Transplant 2012; 12:576 - 89; http://dx.doi.org/10.1111/j.1600-6143.2011.03879.x; PMID: 22176785
- Kwun J, Malarkannan S, Burlingham WJ, Knechtle SJ. Primary vascularization of the graft determines the immunodominance of murine minor H antigens during organ transplantation. J Immunol 2011; 187:3997 - 4006; http://dx.doi.org/10.4049/jimmunol.1003918; PMID: 21900176
- Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol 2011; 11:221 - 30; http://dx.doi.org/10.1038/nri2940; PMID: 21350581
- Streilein JW. Ocular immune privilege: therapeutic opportunities from an experiment of nature. Nat Rev Immunol 2003; 3:879 - 89; http://dx.doi.org/10.1038/nri1224; PMID: 14668804
- Boisgérault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol 2001; 167:1891 - 9; PMID: 11489968
- Huq S, Liu Y, Benichou G, Dana MR. Relevance of the direct pathway of sensitization in corneal transplantation is dictated by the graft bed microenvironment. J Immunol 2004; 173:4464 - 9; PMID: 15383577
- Ueno T, Tanaka K, Jurewicz M, Murayama T, Guleria I, Fiorina P, et al. Divergent role of donor dendritic cells in rejection versus tolerance of allografts. J Am Soc Nephrol 2009; 20:535 - 44; http://dx.doi.org/10.1681/ASN.2008040377; PMID: 19129312
- Ko S, Deiwick A, Jäger MD, Dinkel A, Rohde F, Fischer R, et al. The functional relevance of passenger leukocytes and microchimerism for heart allograft acceptance in the rat. Nat Med 1999; 5:1292 - 7; http://dx.doi.org/10.1038/15248; PMID: 10545996
- Demetris AJ, Murase N, Ye Q, Galvao FH, Richert C, Saad R, et al. Analysis of chronic rejection and obliterative arteriopathy. Possible contributions of donor antigen-presenting cells and lymphatic disruption. Am J Pathol 1997; 150:563 - 78; PMID: 9033271
- Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, et al. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol 2011; 187:2072 - 8; http://dx.doi.org/10.4049/jimmunol.1100762; PMID: 21804023
- Land WG. Emerging role of innate immunity in organ transplantation part III: the quest for transplant tolerance via prevention of oxidative allograft injury and its consequences. Transplant Rev (Orlando) 2012; 26:88 - 102; PMID: 22000661
- Graca L, Cobbold SP, Waldmann H. Identification of regulatory T cells in tolerated allografts. J Exp Med 2002; 195:1641 - 6; http://dx.doi.org/10.1084/jem.20012097; PMID: 12070291
- Li W, Bribriesco AC, Nava RG, Brescia AA, Ibricevic A, Spahn JH, et al. Lung transplant acceptance is facilitated by early events in the graft and is associated with lymphoid neogenesis. Mucosal Immunol 2012; In press http://dx.doi.org/10.1038/mi.2012.30; PMID: 22549742
- Benichou G, Takizawa PA, Olson CA, McMillan M, Sercarz EE. Donor major histocompatibility complex (MHC) peptides are presented by recipient MHC molecules during graft rejection. J Exp Med 1992; 175:305 - 8; http://dx.doi.org/10.1084/jem.175.1.305; PMID: 1730925
- Lechler RI, Lombardi G, Batchelor JR, Reinsmoen NL, Bach FH. The molecular basis of alloreactivity. Immunol Today 1990; 11:83 - 8; http://dx.doi.org/10.1016/0167-5699(90)90033-6; PMID: 2186745
- Akiyama Y, Caucheteux SM, Vernochet C, Iwamoto Y, Tanaka K, Kanellopoulos-Langevin C, et al. Transplantation tolerance to a single noninherited MHC class I maternal alloantigen studied in a TCR-transgenic mouse model. J Immunol 2011; 186:1442 - 9; http://dx.doi.org/10.4049/jimmunol.1003023; PMID: 21178009
- Roelen DL, van Bree FP, van Beelen E, van Rood JJ, Claas FHJ. No evidence of an influence of the noninherited maternal HLA antigens on the alloreactive T cell repertoire in healthy individuals. Transplantation 1995; 59:1728 - 33; http://dx.doi.org/10.1097/00007890-199506270-00015; PMID: 7541578
- Miles CD, Schaubel DE, Liu D, Port FK, Rao PS. The role of donor-recipient relationship in long-term outcomes of living donor renal transplantation. Transplantation 2008; 85:1483 - 8; http://dx.doi.org/10.1097/TP.0b013e3181705a0f; PMID: 18497690
- Burlingham WJ, Jankowska-Gan E, VanBuskirk A, Orosz CG, Lee JH, Kusaka S. Loss of tolerance to a maternal kidney transplant is selective for HLA class II: evidence from trans-vivo DTH and alloantibody analysis. Hum Immunol 2000; 61:1395 - 402; http://dx.doi.org/10.1016/S0198-8859(00)00217-2; PMID: 11163098
- Kusaka S, Grailer AP, Fechner JH Jr., Jankowska-Gan E, Oberley T, Sollinger HW, et al. Clonotype analysis of human alloreactive T cells: a novel approach to studying peripheral tolerance in a transplant recipient. J Immunol 2000; 164:2240 - 7; PMID: 10657680
- Nijagal A, Fleck S, Hills NK, Feng S, Tang Q, Kang SM, et al. Decreased risk of graft failure with maternal liver transplantation in patients with biliary atresia. Am J Transplant 2012; 12:409 - 19; http://dx.doi.org/10.1111/j.1600-6143.2011.03895.x; PMID: 22221561
- Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med 2011; 3:86ra51; http://dx.doi.org/10.1126/scitranslmed.3002093; PMID: 21653831