Abstract
Over many years evidence accumulated that circadian rhythms are related to psychiatric disorders.1-3 However, a mechanistic relationship between the circadian clock and mood related behaviors remained enigmatic. Now, we have reported that monoamine oxidase A (MAOA), a mitochondrial enzyme degrading catecholamines including dopamine, is regulated by components of the circadian clock .4 Interestingly, this regulation is variable depending on cell type, indicating the presence of cell type specific factors modulating BMAL1/NPAS2 or BMAL1/CLOCK dependent transcription. In the mesolimbic dopaminergic reward circuit, including the ventral tegmental area (VTA) and the ventral striatum/nucleus accumbens (NAc) we found a positive influence of Period 2 (PER2) on transcriptional activation of Maoa using mice mutant in the Per2 gene. These animals show less Maoa mRNA expression and MAO activity compared to wild type littermates. This is probably the reason for the observed increase in dopamine levels in the striatum of Per2 mutant mice what leads to alteration in despair-based behavioral tests. These results suggest that clock components can influence dopamine metabolism and mood-related behaviors.
Addendum to:
Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of monoamine oxidase a by circadian-clock components implies clock influence on mood. Curr Biol 2008;18:678-83.
The mammalian circadian clock influences a multitude of physiological processes such as cardiovascular activity, sleep and wakefulness, metabolism and brain function just to name a few. An optimal timing of diverse biochemical processes will not only separate incompatible chemical reactions from each other but also allowS targeted use of energy. Therefore, it is evident that a correctly synchronized circadian clock that is in tune with the environment brings about advantages for an individual to optimally perform in a competitive habitat.Citation5
Correct synchronization of the circadian clock is mainly mediated by light hitting the retina that is directly connected via the retino-hypothalamic tract to the suprachiasmatic nuclei (SCN) harboring the main clock in the brain.Citation6 Light administered in the dark phase activates the clock genes Per1 and Per2, which leads to phase advances or delays, respectively.Citation7–Citation9 Alternatively, limited food can act as a synchronizing signal and override light input.Citation10 These input signals to the clock adapt its phase and lead to an orchestrated physiology that is synchronized with natural events and hence, the organism is optimally prepared for interactions with the environment.
Given the importance of light and food for clock synchronization, changes in life-style, affecting the two afore mentioned parameters, will alter the phase relationship between the internal circadian clock and external geophysical time. Ultimately, this will lead to chronic de-synchronization of the body's physiology leading to depression and obesity, which are observed with increasing frequency in the population of industrialized countries. To find explanations for this mysterious increase in mood-related disorders, we set out to investigate mice with a dysfunctional circadian clock hoping to relate the clock mechanism to mood-related behaviors.
Our studyCitation4 points to a direct involvement of circadian clock components in the transcriptional regulation of monoamine oxidase A. We find that especially dopamine is affected in the mesolimbic dopaminergic system which is important in regulating reward in response to drugs and food. In support of this are our previous findings that mice mutant in the clock component Per2 show altered responses to cocaine,Citation11 increased alcohol consumptionCitation12 and lack of food anticipation.Citation13 Reports from other laboratories also indicate involvement of another clock component, the gene Clock, in the development of obesityCitation14 and the expression of a mania-like phenotype as observed in patients with bipolar disorder.Citation15 Taken together, it appears that circadian clock components affect reward-related behaviors such as feeding and consumption of drugs of abuse.
Recent findings indicate that feeding signals and drugs of abuse share neurobiological mechanisms that act in the midbrain where dopamine is a major neurotransmitter (reviewed in ref. Citation16). In particular leptin appears to reduce the firing rate of dopaminergic neuronsCitation17 indicating that leptin exerts a direct influence on dopamine neurons via leptin receptors in the VTA.Citation18 Interestingly, leptin-deficient mice show reduced levels of dopamine in the NAc and leptin treatment increases the synthesis and activity of tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis.Citation18 Therefore it seems conceivable that leptin, which is increased in Clock mutant miceCitation14 leads to elevated TH activity and dopamine in the VTA observed in these animals.Citation19 Altered leptin levels, however, seem not to be the cause of the elevated dopamine levels detected in the striatum of Per2 mutant animals.Citation4 TH protein levels are not altered in the NAc of these animals (). Furthermore, protein phosphatase 2A (PP2A) that de-phosphorylates and thereby holds TH enzyme in a reduced/inactive stateCitation20 is slightly increased in the striatum (). Hence, possible elevated leptin levels would be compensated by increase in PP2A activity leading to TH activity levels that are comparable between wild type and Per2 mutant mice (). The difference observed between the two genotypes is in the phase of the fluctuation and not in the total levels of TH activity. Therefore, the increased amount of dopamine seen in Per2 mutant animals seems to be mainly caused by the clock via transcriptional regulation of Maoa and unlikely to be an indirect effect via leptin.
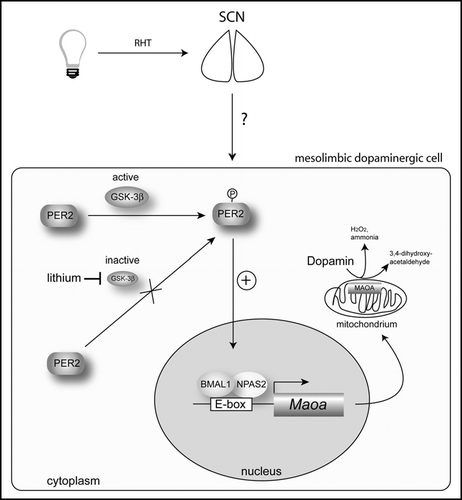
Based on our findingsCitation4 and the fact that PER2 interacts with glycogen synthase kinase-3β (GSK-3β),Citation21 we propose the following model. PER2 phosphorylation by GSK-3β favors accumulation of PER2 in the nucleus. There it enhances the NPAS2/BMAL1-mediated transcription of Maoa and more enzyme is generated that ends up in the inner mitochondrial membrane. MAOA degrades dopamine producing 3,4-dihydroxyacetaldehyde, peroxide and ammonia. Increased levels of PER2 might therefore lead to less dopamine and a more depressed mood state.Citation22,Citation23 The mood stabilizer lithium inhibits the action of GSK-3βCitation24 and less PER2 is phosphorylated.Citation21 As a consequence PER2 becomes less abundant in the nucleus what leads to less MAOA production. As a consequence dopamine will rise and mood status will improve. This might be a potential mechanism explaining the beneficial effects of lithium on mood (). Future studies will show whether this model indeed reflects the actions of lithium. Another important question is how light plays into our model (). Does light affect phosphorylation status of PER2? How is light information that reaches the SCN transmitted to other brain areas such as the mesolimbic dopaminergic system? Does this involve modulation of phosphorylated PER2? The answers to these questions will contribute to the understanding how the known effects of light and lithium on mood are manifested and why the circadian clock might be involved in their actions.
Figures and Tables
Figure 1 Diurnal variation of tyrosine hydroxylase (TH) and Protein Phosphatase 2A (PP2A) in wild type and Per2 mutant mice. (A) TH protein levels in the nucleus accumbens. (B) PP2A activity in the striatum. (C) TH activity in the striatum. Data are plotted as means ± SEM (n = 3 or more). Significant differences were determined by two-way ANOVA combined with Bonferroni posttests and two-tailed unpaired t-test. The white and black bars indicate light and dark phase. Animals were entrained to a 12:12 h LD cycle. Asterisks mark significant differences determined by Bonferroni posttest. Values 0/24 are double plotted.
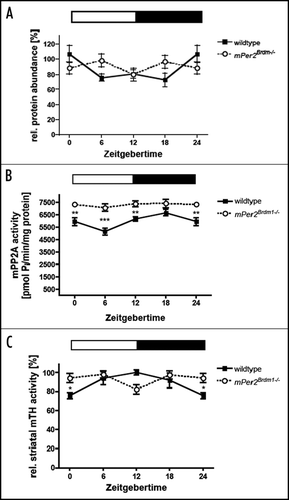
Figure 2 Model of how PER2 affects monoamine oxidase A (Maoa) expression and dopamine levels in the mesolimbic dopaminergic system (for details see text). Light affects the molecular clock mechanism in the suprachiasmatic nuclei (SCN) via the retinohypothalamic tract (RHT), which directly connects the retina of the eye with the SCN. How this signal is transmitted to mesolimbic dopaminergic cells is not known. BMAL1 = brain and muscle arnt-like factor 1; GSK-3β = glycogen synthase kinase-3 β; NPAS2 = neuronal Period-Arndt-Single-minded-domain protein 2; PER2 = period 2.
Acknowledgements
We like to thank Dr. Hui-Chen Lu for comments on the manuscript. This research was supported by the Velux Foundation, the Swiss National Science Foundation and EUCLOCK.
Addendum to:
References
- Wirz-Justice A. [Biological rhythms and depression]. Schweiz Arch Neurol Psychiatr 1986; 137:87 - 96
- Wirz-Justice A, Bucheli C, Schmid AC, Graw P. A dose relationship in bright white light treatment of seasonal depression. Am J Psychiatry 1986; 143:932 - 933
- Benedetti F, Colombo C, Barbini B, Campori E, Smeraldi E. Morning sunlight reduces length of hospitalization in bipolar depression. J Affect Disord 2001; 62:221 - 223
- Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, Brunk I, Spanagel R, Ahnert-Hilger G, Meijer JH, Albrecht U. Regulation of monoamine oxidase a by circadian-clock components implies clock influence on mood. Curr Biol 2008; 18:678 - 683
- Roenneberg T, Merrow M. The network of time: understanding the molecular circadian system. Curr Biol 2003; 13:198 - 207
- Hirota T, Fukada Y. Resetting mechanism of central and peripheral circadian clocks in mammals. Zoolog Sci 2004; 21:359 - 368
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell 1997; 91:1055 - 1064
- Albrecht U, Zheng B, Larkin D, Sun ZS, Lee CC. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms 2001; 16:100 - 104
- Yan L, Silver R. Differential induction and localization of mPer1 and mPer2 during advancing and delaying phase shifts. Eur J Neurosci 2002; 16:1531 - 1540
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 2000; 14:2950 - 2961
- Abarca C, Albrecht U, Spanagel R. Cocaine sensitization and reward are under the influence of circadian genes and rhythm. Proc Natl Acad Sci USA 2002; 99:9026 - 9030
- Spanagel R, Pendyala G, Abarca C, Zghoul T, Sanchis-Segura C, Magnone MC, Lascorz J, Depner M, Holzberg D, Soyka M, Schreiber S, Matsuda F, Lathrop M, Schumann G, Albrecht U. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 2005; 11:35 - 42
- Feillet CA, Ripperger JA, Magnone MC, Dulloo A, Albrecht U, Challet E. Lack of food anticipation in Per2 mutant mice. Curr Biol 2006; 16:2016 - 2022
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 2005; 308:1043 - 1045
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA 2007; 104:6406 - 6411
- Simerly R. Feeding signals and drugs meet in the midbrain. Nat Med 2006; 12:1244 - 1246
- Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006; 51:801 - 810
- Fulton S, Pissios P, Manchon RP, Stiles L, Frank L, Pothos EN, Maratos-Flier E, Flier JS. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006; 51:811 - 822
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, Nestler EJ. Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proc Natl Acad Sci USA 2005; 102:9377 - 9381
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem 2004; 91:1025 - 1043
- Iitaka C, Miyazaki K, Akaike T, Ishida N. A role for glycogen synthase kinase-3beta in the mammalian circadian clock. J Biol Chem 2005; 280:29397 - 29402
- Randrup A, Braestrup C. Uptake inhibition of biogenic amines by newer antidepressant drugs: relevance to the dopamine hypothesis of depression. Psychopharmacology (Berl) 1977; 53:309 - 314
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nat Rev Neurosci 2006; 7:137 - 151
- Klemfuss H. Rhythms and the pharmacology of lithium. Pharmacol Ther 1992; 56:53 - 78