Abstract
Variation in floral traits including odor, color, and morphology, demonstrate the selective pressures imposed by specific pollinator taxa, such as insects and birds. In southern Arizona, Manduca (Sphingidae) hawkmoths are associated with Datura wrightii (Solanaceae) at both the larval (herbivore) and adult (nectar feeding) stages. However during most of the summer Manduca feeds on “bat-adapted” Agave spp. (Agaveacea) flowers, and only use Datura when it is at peak bloom. Manduca’s nectar-host use appears to be mediated through innate odor preferences and olfactory learning; they prefer Datura’s “hawkmoth-adapted” traits, which facilitate the maintenance of their coevolutionary relationship, yet they are flexible enough to explore and learn to utilize novel resources, such as agave. This behavioral flexibility is likely responsible for the frequent observation of generalized, or mixed, pollination systems. Given that Manduca visit agave species in southern Arizona, we hypothesize that the differences in flower phenotype between two closely related agave species may be associated with the importance of hawkmoths relative to bats. The southernmost agave, Agave palmeri (Agavacea), exhibits floral traits typical of bat pollination, whereas the northernmost species, Agave chrysantha (Agavacea), exhibits mixed floral traits which appear to be adapted to insects, and to a lesser extent, bats. The differences between these agaves are likely correlated with the geographic overlap in migratory bats from Mexico and resident hawkmoth populations. Thus D. wrightii, A. palmeri, and A. chrysantha populations represent a unique system in which to examine the evolution of floral traits in both specialized and mixed pollination systems associated with spatially variable pollinator assemblages.
Floral Advertisements and Pollinator Visitation
Floral traits are signals that animal pollinators use to locate important food resources. Flower phenotypes are believed to be non-random combinations of colors, odors and morphologies hypothesized to reflect selective pressure imposed by certain classes of pollinators.Citation1 For example, some of the well-studied examples of floral signals come from plants with nocturnal antithesis thought to reflect adaptations for hawkmoth or bat pollination. Flowers having hawkmoth pollinated traits are white and highly reflective, emit a strong pleasant scent, and have sucrose-dominant nectar.Citation2–Citation4 In contrast, bat pollinated flowers are dull yellow or cream-colored, emit a strong fetid or pungent scent, and have hexose- (fructose and glucose) dominant nectar.Citation5,Citation6 Phylogenetic and morphological studies have demonstrated that these characteristics have evolved independently in many different plant families.Citation7 The convergence of these common floral features makes night blooming plants excellent model systems in which to examine plant-pollinator interactions and the evolution of floral traits.
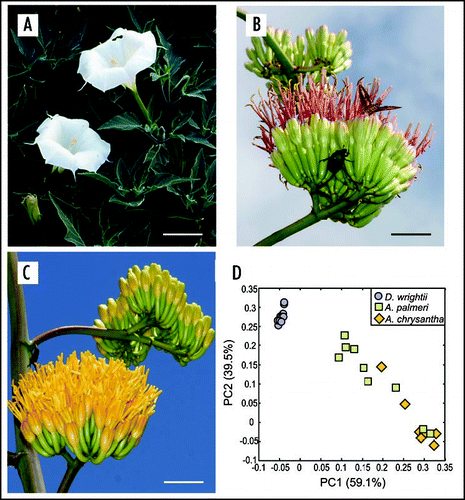
In the semi-arid environments of southern Arizona USA, several hawkmoth and bat-adapted plants occur in sympatry. An example of this is Palmer's Agave (Agave palmeri, Agavaceae), and jimsonweed (Datura wrightii, Solanaceae), which exhibit bat and hawkmoth-adapted floral characters, respectively. D. wrightii floral traits include a sweet smelling odor composed of terpenoids (mono- and sesquit-erpenoids) (79%) and benzenoids (18%), a sucrose-rich nectar, and highly reflective corollas (reflectance > 50%) that appear white to the human eye (). In contrast, A. palmeri traits consist of a foul floral scent dominated by esters (30%), sulfur compounds (<1%), and monoterpenoids (59%), abundant hexose-rich nectar, and a pale dull coloration (reflectance < 25%) (). The hawkmoth, Manduca sexta (Sphingidae), is an important pollinator for D. wrightii and has an innate attraction to D. wrightii's floral odor. M. sexta hawkmoths also visit A. palmeri flowers, but only when D. wrightii is not locally abundant. Moths learn to utilize A. palmeri's abundant nectar resources through olfactory-mediated exploration and learning.Citation4 The ability by pollinators to shift from using one nectar resource to another allows populations to exist when a preferred resource is scarce while likely permitting the maintenance of plant-pollinator associations.
From an evolutionary perspective, behavioral plasticity is likely an adaptation in response to a variable floral environment. Pollinators capable of collecting floral resources from a wide variety of plants, including those morphologically adapted to different pollinator taxa, are more likely to persist within a fluctuating environment compared with those specialized to a single, or few, partner species.Citation8,Citation9 Thus it is not surprising that generalized, or mixed pollination systems, are more common than previously believed.Citation10
Floral Traits of Two Agave Species in the Sonoran Desert
In the Sonoran Desert agaves are regarded as “keystone species” during summer months by providing copious amounts of nectar and pollen for a wide range of insects and vertebrates pollinators.Citation11–Citation14 Two agaves in particular, Agave palmeri and Agave chrysantha, represent a unique system to explore the evolution of floral traits under selection by a diverse pollinator assemblage. While the evolutionary relationship between Agave chrysantha and A. palmeri has yet to be fully resolved using molecular techniques, the occurrence of natural hybrids and morphological studies suggest that they are closely related.Citation15 Agave chrysantha is endemic to central and southern Arizona, and is believed to be a relatively young species, whereas Agave palmeri is believed to be older and has a wider range that extends into Mexico.Citation15,Citation16 The flowers of both agaves superficially resemble one another, possibly reflecting their shared evolutionary history: both produce abundant nectar resources (>100 µl/day), emit odor composed of monoterpenes and aliphatic compounds known to attract diverse insect species, and have floral morphologies that permit nectar access by insects, bats and birds. However, their flowers' differ from one another in several ways. For instance, corollas of A. chrysantha are bright yellow-orange rather than the pale cream color of A. palmeri (), which suggests that the former are visually more adapted to diurnal pollinators, and the latter to nocturnal pollinators.Citation16 A. chrysantha flowers produce nectar that has more than 63-times the sucrose content of A. palmeri (7.56 mg/flower versus 0.12 mg/flower, respectively), which suggest that A. chrysantha's nectar is under greater selection by insect pollinators.
While differing in their visual display and nectar chemistry, examination of their scent profiles by principal components analysis (PCA) revealed substantial overlap in the two agave species (). For instance, A. palmeri and A. chrysantha share similar percentages of chemical odorant classes, including low levels of sulfur compounds (<1%)—a common marker of bat-adapted flowers—esters (ca. 53% and 77%, respectively), and terpenoids (ca. 38% and 15%, respectively). The contrast in floral color and nectar chemistry suggest that these two agaves are adapted to different pollinator assemblages, but the similarity in their floral odors likely reflects their shared evolutionary history.
A Unique Study System for Evaluating Pollinator-Mediated Floral Evolution
Assuming that A. chrysantha recently evolved from A. palmeri,Citation15 the differences in floral color, nectar and odor chemistry between the two species are likely due to differences in visitation by migratory bats, in particular the migratory Lesser Long-nosed Bat (Leptonycteris curasoae). Recent workCitation12,Citation14,Citation17 suggests that the relationship between Lesser Long-nosed Bats and their nectar plants varies latitudinally, with bats and plants mutually dependent upon each other in central Mexico, but plants are less depended upon bats at the northern end of their range (i.e., Arizona, USA), presumably because bat migrations are variable from year to year. Given that the range of the Lesser Long-nosed Bat entirely overlaps with the distribution of A. palmeri in southern Arizona, but only overlaps the southern most portion of A. chrysantha's range,Citation14 it is likely that A. chrysantha evolved in the absence of bat pollination. Coincidentally, the region where A. chrysantha and Lesser Long-nosed Bat's ranges overlap is where A. chrysantha hybridizes with A. palmeri, suggesting that bat-mediated gene flow, or the lack thereof, is central to the evolution of A. chrysantha's floral characters.
The differences in floral traits likely reflect variation in the effective pollinators of these two agave species. Sulfur compounds are important mediators of bat attraction to flowersCitation18 and it has been suggested that shifts from bat to moth pollination reflects the reduction of sulfur compounds and the presence of terpenoids and benzenoids in a floral headspace. For instance, flowers of the plant Browneopsis disepala (Leguminosae) are visited by both moths and bats and displays a mixed pollination syndrome with a bat pollinated floral display and an odor profile that is dominated by aliphatics and monoterpenes but lacks the sulfur compounds.Citation7 These agaves represent additional examples of mixed pollination systems, wherein the odor profile and visual displays of A. palmeri and A. chrysantha indicate that one is more bat-adapted than the other. Because hawkmoths are common nocturnal visitors of these agave species,Citation14–Citation16 the agaves, together with D. wrightii which is specialized for hawkmoth pollination, present an excellent system to examine the relationship between floral advertisements and rewards with their primary nocturnal visitors.
Figures and Tables
Figure 1 Contrast in floral characterisitics between D. wrightii, A. palmeri and A. chrysantha plants. The three plants are visited by nocturnal hawkmoths, but the floral traits in each plant differ from one another. (A) D. wrightii flowers. Note the bright floral reflectance of the corollas that produces a contrast with the dark foliage background. (B) A. palmeri umbel. Each umbel contains 8–20 flowers. Hyles lineata (Sphingidae), and Cotinus mutabilis (Scarabaeidae), are feeding from the umbel. (C) A. chrysantha umbel. The morphology of A. chrysantha closely resembles that of A. palmeri, but note the bright yellow corollas. (D) Principal-components analysis of the floral odor from these three plants. The D. wrightii flowers (blue circles) group together. In contrast, the agaves appear more variable. The odor of A. palmeri flowers (green squares) appears to more closely resemble that of D. wrightii due to the presence of monoterpenes and benzenoids in its headspace, but some A. palmeri flowers are also grouped in the same PCA space of A. chrysantha (yellow diamonds) due to their aliphatic esters. Scale bar = 5 cm. Picture images courtesy of Charles Hedgecock RBP, FBCA (ARL Division of Neurobiology, University of Arizona) and T. Beth Kinsey.
Addendum to:
References
- Faegri K, van der Pijl L. The principles of pollination ecology 1979; Oxford, UK Pergamon Press
- Grant V. Behavior of hawkmoths on flowers of Datura metaloides. Bot Gazette 1983; 144:439 - 449
- Raguso RA, Henzel C, Buchman SL, Nabhan GP. Trumpet flowers of the Sonoran desert: floral biology of peniocereus cacti and sacred datura. I J Plant Sci 2003; 164:877 - 892
- Riffell JA, Alarcon R, Abrell L, Davidowitz G, Bronstein JL, Hildebrand JG. Behavioral consequences of innate preferences and olfactory learning in hawkmoth-flower interactions. Proc Natl Acad Sci USA 2008; 105:3404 - 3409
- Bestmann HJ, Winkler L, von Helversen O. Headspace analysis of volatile flower scent constituents of bat-pollinated plants. Phytochemistry 1997; 46:1169 - 1172
- Dobson HEM. Dudareva N, Pichersky E. Relationship between floral fragrance composition and type of pollinator. Biology of floral scent 2006; Boca Raton, FL CRC Press 147 - 198
- Knudsen JT, Tollsten L. Floral scent in bat-pollinated plants: a case for convergent evolution. Bot J Linn Soc 1995; 119:45 - 57
- Memmott J, Waser NM, Price MV. Tolerance of pollination networks to species extinctions. Proc R Soc B 2004; 271:2605 - 2611
- Waser NM, Chittka L, Price MV, Williams NM, Ollerton J. Generalization in pollination systems, and why it matters. Ecology 1996; 77:1043 - 1060
- Waser NM. Waser NM, Ollerton J. Specialization and generalization in plant-pollinator interactions: A historical perspective. Plant-pollinator interactions: From specialization to generalization 2006; Chicago, IL University of Chicago Press 3 - 17
- Molina-Freaner F, Eguiarte LE. The pollination biology of two paniculate agaves (Agavaceae) from northwestern Mexico: contrasting roles of bats as pollinators. Am J Bot 2003; 90:1016 - 1024
- Scott PE. Timing of Agave palmeri flowering and nectar-feeding bat visitation in the Peloncillos and Chiricahua mountains. Southwestern Nat 2004; 49:425 - 434
- Silva-Montellano A, Eguiarte LE. Geographic patterns in the reproductive ecology of Agave lechuguilla (Agavaceae) in the Chihuahuan desert I. Floral characteristics, visitors and fecundity. Am J Bot 2003; 90:377 - 387
- Slauson LA. Pollination biology of two chiropterophilous agaves in Arizona. Am J Bot 2000; 87:825 - 836
- Gentry HS. The agaves of continental North America 1982; Tucson, AZ University of Arizona Press
- Slauson LA. DeBano LF. Factors affecting the distribution, pollination ecology and evolution of Agave chrysantha Peebles and Agave palmeri Engelm. (Agavaceae). Biodiversity and the Management of the Madrean Archipelago: The Sky Islands of Southwestern USA and Northwestern Mexico 1995; USDA Forest Service 194 - 205
- Fleming TH. Nabhan GP. Nectar corridors: migration and the annual cycle of lesser long-nosed bats. Conserving Migratory Pollinators and Nectar Corridors in Western North America 2004; Tucson, AZ University of Arizona Press 23 - 42
- von Helversen O, Winkler L, Bestmann HJ. Sulphur-containing “perfumes” attract flower-visiting bats. J Comp Physiol A 2000; 186:143 - 153