Abstract
Members of the tetraspanin family of transmembrane proteins including CD9, CD37, CD53, CD63, CD81, CD82, CD151 etc. contribute to the structural organization of the plasma membrane by forming microdomain structures, influencing cell fusion, and regulating cell motility. Interestingly, K41, a CD9-specific monoclonal antibody (mAb), inhibits the release of human immunodeficiency virus (HIV-1), and the canine distemper virus (CDV)-, but not measles virus (MV)-induced cell-cell fusion. This mAb, which recognizes a conformational epitope on the large extracellular loop (LEL) of CD9, induced rapid relocation and clustering of CD9 in net-like structures at cell-cell contact areas (1). High-resolution analyses revealed that CD9 clustering is accompanied by the formation of microvilli that protrude from either side of adjacent cell surfaces, thus forming structures like microvilli zippers. While the cellular CD9-associated proteins β1-integrin and EWI-F were co-clustered with CD9 at cell-cell interfaces, viral proteins in infected cells were differentially affected. MV envelope proteins were detected within, whereas CDV proteins were excluded from CD9 clusters, and thus, the tetraspanin CD9 can regulate cell-cell fusion by controlling the access of the viral fusion machinery to cell contact areas.
Laterally interacting tetraspanins in the plasma membrane form, together with other cell membrane receptors, tetraspanin-enriched microdomains (TEMs), also called the tetraspanin web.Citation2–Citation6 Within TEMs, CD9 interacts with β1-integrins, pro-HB-EGF, EWI-2, EWI-F, FPRP, PSG17, and other cell surface proteins,Citation7–Citation11 regulates the plasticity of the cellular membrane, and influences the cell motility and cell fusion.Citation5,Citation12–Citation17 In addition, CD9 modulates also various virus-induced processes at membranes such as membrane fusion, viral budding and release.Citation18–Citation24 Another tetraspanin, CD63, was found to be incorporated into HIV particlesCitation25–Citation27 and to colocalize with CD9 and HIV-1 Gag-proteins in TEMs on the cell surface.Citation24 Treatment of cells with mAb K41 induced the clustering of CD9 and also other tetraspanins (CD63, CD81) and inhibited HIV-1 release suggesting that normal non-aggregated tetraspanin-containing microdomains may be important for egress of the virus. Interestingly, the release of another enveloped virus, influenza virus, was not affected by disruption of TEMs by mAb K41.Citation28
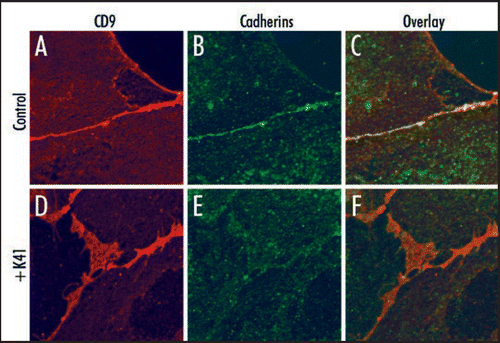
In the case of canine distemper virus (CDV), mAb K41 inhibited the virus-induced cell-cell fusion and virus release, whereas virus uptake was not affected.Citation21,Citation22 The extracellular domain of the viral envelope protein hemagglutinin (H) of CDV determines the susceptibility of the cell-cell fusion to certain CD9-antibodies, however does not itself bind to CD9.Citation29 This suggested that structural alterations of the plasma membrane influencing the activity and/or spatial expression pattern of receptors are involved. We found recently that K41 induces a rapid relocation and clustering of CD9 at cell contact sites, which is associated with the formation of microvilli-like protrusions between contacting cells.Citation1 Co-localization of the other tetraspanin family members CD63, CD81 and CD82 and proteins like EWI-F with CD9 in K41-induced clusters suggested that this mAb can alter the overall organization of surface TEMs. Whereas some cell surface receptors are, others are not affected by the relocation and structural reorganization. In fact, cadherins were found enriched in the thin cell contacts formed in cells before treatment with K41, and after treatment were found less dense in the CD9-enriched net like structures (). In infected cells, the relocation of virus receptors may lead either to a physical separation of the viral fusion machinery from cell-cell contact areas, or to the inclusion of viral envelope proteins, depending on the virus investigated. Cell-cell fusion may result in giant cell formation, as observed in vivo during measles, or may be restricted to a transient micro fusion pore or virological synapse. Since viruses within a host often exploit this contact dependent way for cell-to-cell spread, CD9 may play an important role in viral pathogenesis.
After K41 interaction, the relocation and clustering of CD9 is a relatively fast event occurring within 1–2 h, whereas the formation of microvilli-like protrusions begins with the appearance of the characteristic large net-like CD9-positive structures after 2–3 h and proceeds over a longer period up to 20 h. The mechanism of protrusion formation at CD9-enriched membranes remains unclear. CD9-K41-complexes may bend the membrane into globular structures as detected in the laser scanning microscope and SEM, and together with the underlying actin cytoskeleton this may provide platforms for the formation of microvilli. Interestingly, the protrusions are observed exclusively at cell-cell contact areas, and not on contact-free surface membranes. They resemble those actin based “zippers” or “Velcro”-like structures described for epithelial cell layer repair during development and wound repair (reviewed in ref. Citation30). Our finding that upon treatment with latrunculin B the actin cytoskeleton is disrupted, microvilli zippers collapse and the cells retract from contacts, underlines that the cytoskeleton plays an important role in formation of these microvilli zippers. However, we could not detect actin fibers within the protrusions induced by K41, and it is not known whether they are involved in cell adhesion.
Beyond the tetraspanins, CD9 has predominantly been associated with cell motility and membrane fusion. CD9 enables the gamete-sperm fusion and CD9-deficiency results in reduced female fertility.Citation13–Citation15,Citation31 However, CD9 may also inhibit cell fusion under certain circumstances as demonstrated for mononuclear phagocytes.Citation32 What is the role of CD9 in virus induced cell fusions and cell-to-cell spread of viruses? Interestingly, adverse effects of antibodies to CD9 have been described for the cell-cell fusion induced by CDV, MV and HIV-1. While certain CD81 and CD9 antibodies enhance HIV-induced cell fusion,Citation20 other antibodies such as K41 inhibit HIV budding and release of virus particles. Our results now nicely explained the specific effects on the CDV-induced and lack of effect on the MV-induced cell fusion, and suggested corresponding consequences for other viruses depending on whether receptors are included or excluded from TEMs.
The causative relationship between CD9 aggregation and the formation of microvilli-like protrusions remains unresolved. CD9- and CD81-associated transmembrane receptors such as EWI-proteins, members of a novel subfamily of the Ig-superfamily,Citation7,Citation10,Citation33 may provide mechanistic suggestions. They contain a stretch of basic charged amino acids in their cytoplasmic domains that interacts with ezrin-radixin-moesin (ERM) proteins, and acts as linker to connect TEMs with the actin cytoskeleton.Citation34 We observed that EWI-F expressed on the cell surface co-clustered with CD9 at cell-cell contact areas in response to K41 treatment, and may there provide the link to the actin cytoskeleton. Since we have no experimental support that signal transduction and remodeling of the cytoskeleton may drive the clustering, it is more likely that the antibody interaction on the cell surface is the driving force. The high antibody concentration necessary to induce CD9 clustering (5–15 µg/ml) supports this suggestion. Further investigations are required to elucidate the function of actin, ERM proteins, and signaling for microvilli formation at the sites of CD9 clustering. Interestingly, CD9 is also enriched on microvillar membranes of oocytes and regulates their shape, distribution, and clustering with other tetraspanins and still unknown surface proteins involved in human and mouse gamete fusion.Citation35,Citation36 Our findings underscore the relevance of CD9 for healthy and pathogenic cell-cell fusion processes, and may open interesting strategies to influence the cell-to-cell spread or release of specific viruses.
Figures and Tables
Figure 1 Cell surface localization of cadherins and CD9. Vero cells were not (A–C), or were (D–F) preincubated with 10 µg/ml mAb K41 for 2 h at 37°C. Expression of CD9 was detected using mAb K41 and secondary Alexa-594-conjugated antibodies (red, A and D). Expression of cadherins was detected using pan-cadherin antibodies (Cell Signaling Technology) and secondary Alexa-488 antibodies (green, B and E). The overlay (C and F) reveals a colocalization of cadherins and CD9 predominantly in the absence of K41-preincubation at the cell border (C). The larger CD9-positive net-like structures at cell contact areas as detected in (D) do not contain enriched amounts of cadherins.
Addendum to:
References
- Singethan K, Muller N, Schubert S, Luttge D, Krementsov DN, Khurana SR, Krohne G, Schneider-Schaulies S, Thali M, Schneider-Schaulies J. CD9 clustering and formation of microvilli zippers between contacting cells regulates virus-induced cell fusion. Traffic 2008; 9:924 - 935
- Berditchevsky F. Complexes of tetraspanins with integrins: more than meets the eye. J Cell Sci 2001; 114:4143 - 4151
- Boucheix C, Rubinstein E. Tetraspanins. Cell Mol Life Sci 2001; 58:1189 - 1205
- Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol 2005; 5:136 - 148
- Hemler ME. Tetraspanin proteins mediate cellular penetration, invasion and fusion events and define a novel type of membrane microdomain. Annu Rev Cell Dev Biol 2003; 19:397 - 422
- Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol 2005; 6:801 - 811
- Charrin S, Le Naour F, Oualid M, Billard M, Faure G, Hanash SM, Boucheix C, Rubinstein E. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J Biol Chem 2001; 276:14329 - 14337
- Ellerman DA, Ha C, Primakoff P, Myles DG, Dveksler GS. Direct binding of the ligand PSG17 to CD9 requires a CD9 site essential for sperm-egg fusion. Mol Biol Cell 2003; 14:5098 - 5103
- Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphtheria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin alpha3beta1 at cell-cell contact sites. J Cell Biol 1995; 129:1691 - 1705
- Stipp CS, Kolesnikova TV, Hemler ME. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J Biol Chem 2001; 276:40545 - 40554
- Stipp CS, Orlicky D, Hemler ME. FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J Biol Chem 2001; 276:4853 - 4862
- Baudoux B, Castanares-Zapatero D, Leclerq-Smekens M, Berna N, Poumay Y. The tetraspanin CD9 associates with the integrin alph6beta4 in cultured human epidermal keratinocytes and is involved in cell motility. Eur J Cell Biol 2000; 79:41 - 51
- Kaji K, Oda S, Shikano T, Ohnuki T, Uematsu Y, Sakagami J, Tada N, Miyazaki S, Kudo A. The gamete fusion process is defective in eggs of CD9-deficient mice. Nat Genet 2000; 24:279 - 282
- Le Naour F, Rubinstein E, Jasmin C, Prenant M, Boucheix C. Severely reduced female fertility in CD9-deficient mice. Science 2000; 287:319 - 321
- Miyado K, Yamada G, Yamada S, Hasuwa H, Nakamura Y, Ryu F, Suzuki K, Kosai K, Inoue K, Ogura A, Okabe M, Mekada E. Requirement of CD9 on the egg plasma membrane for fertilization. Science 2000; 287:321 - 324
- Tachibana I, Hemler ME. Role of transmembrane 4 superfamily (TM4SF) proteins CD9 and CD81 in muscle cell fusion and myotube maintenance. J Cell Biol 1999; 146:893 - 904
- Tanio Y, Yamazaki H, Kunisada T, Miyake K, Hayashi SI. CD9 molecule expressed on stromal cells is involved in osteoclastogenesis. Exp Hematol 1999; 27:853 - 859
- Deneka M, Pelchen-Matthews A, Byland R, Ruiz-Mateos E, Marsh M. In macrophages, HIV-1 assembles into an intracellular plasma membrane domain containing the tetraspanins CD81, CD9 and CD53. J Cell Biol 2007; 177:329 - 341
- de Parseval A, Lerner DL, Borrow P, Willett BJ, Elder JH. Blocking of feline immunodeficiency virus infection by a monoclonal antibody to CD9 is via inhibition of virus release rather than interference with receptor binding. J Virol 1997; 71:5742 - 5749
- Gordon-Alonso M, Yanez-Mo M, Barreiro O, Alvarez S, Munoz-Fernandez MA, Valenzuela-Fernandez A, Sanchez-Madrid F. Tetraspanins CD9 and CD81 modulate HIV-1-induced membrane fusion. J Immunol 2006; 177:5129 - 5137
- Löffler S, Lottspeich F, Lanza F, Azorsa DO, ter Meulen V, Schneider-Schaulies J. CD9, atetraspan transmembrane protein, renders cells susceptible to canine distemper virus. J Virol 1997; 71:42 - 49
- Schmid E, Zurbriggen A, Gassen U, Rima B, ter Meulen V, Schneider-Schaulies J. Antibodies to CD9, a tetraspan transmembrane protein, inhibit canine distemper virus (CDV)-mediated cell-cell fusion, but not virus-cell fusion. J Virol 2000; 74:7554 - 7561
- Willett BJ, Hosie MJ, Shaw A, Neil JC. Inhibition of feline immunodeficiency virus infection by CD9 antibody operates after virus entry and is independent of virus tropism. J Gen Virol 1997; 78:611 - 618
- Nydegger S, Khurana S, Krementsov DN, Foti M, Thali M. Mapping of tetraspanin-enriched microdomains that can function as gateways for HIV-1. J Cell Biol 2006; 173:795 - 807
- Gluschankof P, Mondor I, Gelderblom HR, Sattenau QJ. Cell membrane vesicles are major contaminant of gradient-enriched human immunodficiency virus type-1 preparations. Virology 1997; 230:125 - 133
- Meerloo T, Sheikh MA, Bloem AC, de Ronde A, Schutten M, van Els CA, Roholl PJ, Joling P, Goudsmit J, Schuurman HJ. Host cell membrane proteins on human immunodeficiency virus type 1 after in vitro infection of H9 cells and blood mononuclear cells. An immunoelectron microscopic study. J Gen Virol 1993; 74:129 - 135
- Orentas RJ, Hildreth JE. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res Hum Retroviruses 1993; 9:1157 - 1165
- Khurana S, Krementsov DN, de Parseval A, Elder JH, Foti M, Thali M. Human immunodeficiency virus type 1 and influenza virus exit via different membrane microdomains. J Virol 2007; 81:12630 - 12640
- Singethan K, Topfstedt E, Schubert S, Duprex WP, Rima BK, Schneider-Schaulies J. CD9-dependent regulation of Canine distemper virus-induced cell-cell fusion segregates with the extracellular domain of the haemagglutinin. J Gen Virol 2006; 87:1635 - 1642
- Jacinto A, Martinez-Arias A, Martin P. Mechanisms of epithelial fusion and repair. Nat Cell Biol 2001; 3:117 - 123
- Kaji K, Kudo A. The mechanism of sperm-oocyte fusion in mammals. Reproduction 2004; 127:423 - 429
- Takeda Y, Tachibana I, Miyado K, Kobayashi M, Miyazaki T, Funakoshi T, Kimura H, Yamane H, Saito Y, Goto H, Yoneda T, Yoshida M, Kumagai T, Osaki T, Hayashi S, et al. Tetraspanins CD9 and CD81 function to prevent the fusion of mononuclear phagocytes. J Cell Biol 2003; 161:945 - 956
- Clark KL, Zeng Z, Langford AL, Bowen SM, Todd SC. PGRL is a major CD81-associated protein on lymphocytes and distinguishes a new family of cell surface proteins. J Immunol 2001; 167:5115 - 5121
- Sala-Valdes M, Ursa A, Charrin S, Rubinstein E, Hemler ME, Sanchez-Madrid F, Yanez-Mo M. EWI-2 and EWI-F link the tetraspanin web to the actin cytoskeleton through their direct association with ezrin-radixin-moesin proteins. J Biol Chem 2006; 281:19665 - 19675
- Runge KE, Evans JE, He ZY, Gupta S, McDonald KL, Stahlberg H, Primakoff P, Myles DG. Oocyte CD9 is enriched on the microvillar membrane and required for normal microvillar shape and distribution. Dev Biol 2007; 304:317 - 325
- Ziyyat A, Rubinstein E, Monier-Gavelle F, Barraud V, Kulski O, Prenant M, Boucheix C, Bomsel M, Wolf JP. CD9 controls the formation of clusters that contain tetraspanins and the integrin alpha6beta1, which are involved in human and mouse gamete fusion. J Cell Sci 2006; 119:416 - 424