Abstract
Cystine-glutamate transporters import cystine into cells for glutathione synthesis and protection from oxidative stress, but also export significant amounts of glutamate. Increasing evidence suggests that ‘ambient extracellular glutamate’ secreted by cystine-glutamate transporters in the nervous system modulates glutamatergic synapse strength and behavior. To date, the only cystine-glutamate transporter mutants examined behaviorally are Drosophila genderblind mutants. These animals contain loss-of-function mutations in the ‘genderblind’ gene, which encodes an xCT subunit essential for cystine-glutamate transporter function. Genderblind was named based on a mutant courtship phenotype: male genderblind mutants are attracted to normally aversive male pheromones and thus court and attempt to copulate with both male and female partners equally. However, genderblind protein is expressed in many parts of the fly brain and thus might be expected to also regulate other behaviors, including behaviors not related to male courtship or chemosensation. Here, we show that genderblind mutants display faster recovery and increased negative geotaxis after strong mechanical stimuli (e.g. they climb faster and farther after vial banging). This phenotype is displayed by both males and females, consistent with strong genderblind expression in both sexes.
Addendum to: Yael Grosjean, Micheline Grillet, Hrvoje Augustin, Jean-Francois Ferveur, and David E. Featherstone. A glial amino acid transporter controls synapse strength and courtship in Drosophila. Nat Neurosci 2008; 11:54-61.
and
Hrvoje Augustin, Yael Grosjean, Kaiyun Chen, Qi Sheng, and David E. Featherstone Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci 2007; 27:111-23.
Cystine-glutamate transporters are transmembrane proteins that bring cystine into cells and secrete glutamate.Citation1–Citation6 They are heterodimeric proteins composed of ‘heavy’ 4F2hc subunits, and ‘light’ xCT subunits.Citation1,Citation7 4F2hc subunits are thought to regulate protein trafficking, and are utilized by several different types of amino acid transporter. xCT subunits, in contrast, are required for amino acid selectivity and transport, and are found only in cystine-glutamate transporters.Citation1,Citation8–Citation11
Cystine uptake by xCT is important for glutathione synthesis and protection from oxidative stress.Citation12–Citation14 Glutamate export by xCT increases ambient extracellular glutamate.Citation15–Citation17 In the nervous system, where xCT proteins are most highly expressed, altered ambient extracellular glutamate has important functional consequences. For example, cystine-glutamate transport is strongly upregulated in gliomas.Citation3 This causes glutamate excitoxicity and neurodegeneration of surrounding brain tissue, which provides room for tumor growth.Citation18–Citation20 In nonpathological conditions, ambient extracellular glutamate is sufficient to alter glutamate receptor function. Pharmacological inhibition of cystine-glutmate transport in rats decreases tonic activation of metabotropic glutamate receptors and alters drug behavior.Citation21–Citation23 Ambient extracellular glutamate also causes constitutive desensitization of ionotropic glutamate receptors, which suppresses glutamatergic neurotransmission.Citation16,Citation24 This latter role for xCT is exemplified by Drosophila genderblind.
Genderblind (GB) is a Drosophila xCT protein expressed in glia throughout the peripheral and central nervous system.Citation16,Citation25 Ambient extracellular glutamate in Drosophila genderblind mutants is reduced to approximately one-half normal,Citation16,Citation17 and the number of functional synaptic glutamate receptors increases 200–300%.Citation16 Genderblind was named based on the observation that mutant males are attracted to normally aversive male pheromones, and thus court and attempt to copulate with both females and other males equally.Citation25 Consistent with the idea that genderblind regulates behavior by suppressing glutamatergic transmission, the homosexual courtship phenotype could be replicated and/or reversed by genetic and pharmacological manipulation of glutamatergic synapse strength independent of genderblind.Citation25 For example, genderblind mutant males fed apple juice laced with gamma-D-glutamylglycine (γ-DGG), a competitive glutamate receptor antagonist, reverted to normal courtship within 24 hours. Wildtype males fed concanavalin A (a glutamate receptor desensitization inhibitor), on the other hand, began courting other males. Similarly, overexpression of the Drosophila vesicular glutamate transporter, which overloads synaptic vesicles with glutamate and increases synaptic strength, replicated the genderblind mutant phenotype.
Neither genderblind nor glutamate receptors are restricted to regions of the fly brain controlling pheromone processing or court-ship.Citation16,Citation25–Citation28 Furthermore, both male and female Drosophila express genderblind equally.Citation16 Therefore, it seems likely that genderblind mutants might show additional behavioral phenotypes that are not associated with courtship or gender.
Because genderblind suppresses glutamatergic transmission, we reasoned that genderblind mutant behaviors might most often represent an overreaction to sensory stimuli. Drosophila display prominent negative geotaxis, and prefer residing on vertical surfaces. When placed in vials, Drosophila quickly climb the sides. If the bottom of a vial containing flies is brought forcefully into contact with a hard surface, flies are dislodged from the sides and fall to the bottom of the vial. Normally, flies quickly right themselves after being knocked down and immediately begin climbing the sides of the vial, where they eventually distribute themselves. Flies that do not recover well after being knocked to the bottom of the vial are typically referred to as ‘bang-sensitive’ or ‘stress sensitive’. Bang sensitive mutations typically disrupt neuronal excitability or mitochondrial function.Citation29–Citation34
We examined genderblind mutants for bang-sensitive phenotypes. Specifically, we compared homozygous gb[KG07905] flies to precise excision and ‘Oregon R’ wildtype controls. gb[KG07905] mutants carry a transposon insertion in the 5′ end of the genderblind gene, which reduces genderblind transcript and protein levels to 53% and 35% of normal, respectively.Citation25 In ‘precise excision flies’, the gb[KG07905] transposon insertion has been cleanly excised (verified by sequencing).Citation16,Citation25 Precise excision flies therefore control for genetic background.
To test bang recovery, four flies of the same gender were placed in an empty polystyrene Drosophila vial (95 mm tall x 24 mm diameter), upon which was marked a line 7 cm from the bottom. The vial was manually banged twice, quickly and firmly, on a slightly padded surface (computer mouse mat). This caused all flies to fall to the bottom of the vial, after which they quickly righted themselves and climbed toward the top. Neither genderblind mutants nor control flies showed any apparent seizures or immobilization characteristic of bang-sensitive mutants. We recorded the number of flies that climbed past the 7 cm ‘finish line’ before stopping, along with the speed of each fly that crossed the line.
As shown in , males and females (2–6 days after eclosion) climbed vials at the same speed. However, gb[KG07905] mutant flies climbed the vials approximately three times faster than either wildtype or precise excision controls (). When the percentage of flies crossing the finish line was compared, we noted that a higher percentage of wildtype females crossed the line compared to wildtype males, suggesting some natural gender difference in geotaxic behavior. However, precise excision controls did not show this gender difference, and the largest difference was attributable to loss of genderblind, as significantly more gb[KG07905] flies crossed the finish line compared to wildtype or precise excision controls, regardless of gender (). Faster climbing by gb[KG07905] suggests that genderblind mutants might locomote faster. But gb[KG07905] mutants do not show faster locomotion, compared to controls.Citation25 Consistent with this, we noted that the upward trajectory of gb[KG07905] flies was straighter than that of control flies, which tended to ‘wander’ slightly more on their way to the finish line. Genderblind mutants in vials at rest also do not obviously distribute themselves differently on the sides of the vial, suggesting that these climbing differences do not represent alterations in negative geotaxis. The accelerated bang recovery in genderblind mutants may therefore represent enhanced goal-directed activity (assuming climbing back up the vial after being knocked to the bottom represents a ‘goal’).
To determine whether repeated banging might amplify differences between genderblind mutants and control flies, we tested groups of flies three consecutive times in three minutes (). Males and females were tested separately, but measurements were combined for graphing () and statistical comparisons. As shown (), there was no obvious trend in either climbing speed or the fraction of flies climbing past the 7 cm line during the three tests.
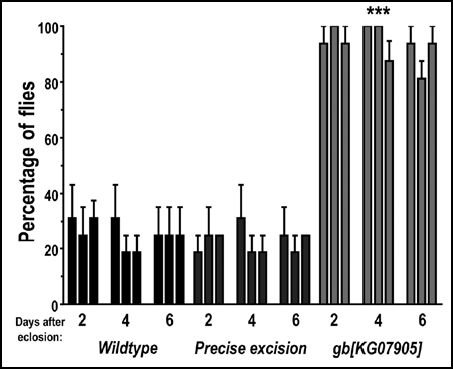
Age-related changes in geotaxis have been observed in Drosophila.Citation35 To test whether changes in gb[KG07905] climbing behavior might be at least partially attributable to or enhanced by age-related geotaxis differences, we measured the number of flies crossing the finish line in each genotype 2, 4 or 6 days after eclosion (). As for , males and females were tested separately, but measurements were combined for presentation and statistical comparison. We observed no effect of age on the behavior within this range; gb[KG07905] mutants always crossed the line with higher frequency compared to controls ().
The accelerated bang recovery and climbing shown here for gb[KG07905] mutants is likely attributable to stronger glutamatergic transmission, as demonstrated for gb[KG07905] male homosexual behavior.Citation25 The glutamatergic circuits that control courtship in flies have yet to be worked out, and developmental alterations associated with mutant courtship remain controversial.Citation36 The accelerated bang recovery and climbing described here are therefore very useful in that they increase the number of specific circuits available for analysis. Our findings also support the idea that both male and female genderblind mutants have altered brain function, consistent with the fact that genderblind is expressed in both sexes.
Figures and Tables
Figure 1 Genderblind mutants recover from banging and climb faster and higher than controls, irrespective of gender. After vials containing groups of four male or four female flies were banged twice to knock flies to the bottom, the speed and number of flies reaching a 7 cm high ‘finish line’ were recorded. Consistently more gb[KG07905] flies passed the line, compared to controls, and did so faster. The height of each bar in the graph represents the average of 9–53 measurements (top) or 23–30 measurements (bottom), from approximately 20 different flies. Error bars represent S.E.M. Asterisks represent statistical significance where p < 0.001.
![Figure 1 Genderblind mutants recover from banging and climb faster and higher than controls, irrespective of gender. After vials containing groups of four male or four female flies were banged twice to knock flies to the bottom, the speed and number of flies reaching a 7 cm high ‘finish line’ were recorded. Consistently more gb[KG07905] flies passed the line, compared to controls, and did so faster. The height of each bar in the graph represents the average of 9–53 measurements (top) or 23–30 measurements (bottom), from approximately 20 different flies. Error bars represent S.E.M. Asterisks represent statistical significance where p < 0.001.](/cms/asset/225bda6e-23ec-416f-b481-4bb4ee0ad578/kcib_a_10906437_f0001.gif)
Figure 2 Genderblind mutants recover from banging and climb faster and higher than controls during repeated trials. As for , vials containing groups of four male or four female flies were banged twice to knock flies to the bottom, and the speed and number of flies reaching a 7 cm high ‘finish line’ were recorded. This test was repeated three times in three consecutive minutes for each group. As shown, consistently more gb[KG07905] flies passed the line, compared to controls, and did so faster, regardless of trial number. The height of each bar in the graph represents the average of ten measurements from 20 males and 20 females. Error bars represent S.E.M. Asterisks represent statistical significance where p < 0.001.
![Figure 2 Genderblind mutants recover from banging and climb faster and higher than controls during repeated trials. As for Figure 1, vials containing groups of four male or four female flies were banged twice to knock flies to the bottom, and the speed and number of flies reaching a 7 cm high ‘finish line’ were recorded. This test was repeated three times in three consecutive minutes for each group. As shown, consistently more gb[KG07905] flies passed the line, compared to controls, and did so faster, regardless of trial number. The height of each bar in the graph represents the average of ten measurements from 20 males and 20 females. Error bars represent S.E.M. Asterisks represent statistical significance where p < 0.001.](/cms/asset/8b98d7ec-8997-45b1-834c-b979f50f01ae/kcib_a_10906437_f0002.gif)
Figure 3 Genderblind mutants recover from banging and climb faster and higher than controls, irrespective of age (within 2–6 days after eclosion). Flies were tested as described for , at age = 2, 4 or 6 days after eclosion (emergence from the pupa). The height of each bar in the graph represents the average of ten measurements from 20 males and 20 females. Error bars represent S.E.M. Asterisks represent statistical significance where p < 0.001.
Acknowledgements
Funding for this work was provided by grants from the Muscular Dystrophy Association and US National Institute of Neurological Disorders and Stroke (R01NS045628) to David E. Featherstone.
Addendum to:
References
- Sato H, Tamba M, Ishii T, Bannai S. Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. J Biol Chem 1999; 274:11455 - 11458
- Sato H, Tamba M, Kuriyama-Matsumura K, Okuno S, Bannai S. Molecular cloning and expression of human xCT, the light chain of amino acid transport system xc. Antioxid Redox Signal 2000; 2:665 - 671
- Kim JY, Kanai Y, Chairoungdua A, Cha SH, Matsuo H, et al. Human cystine/glutamate transporter: cDNA cloning and upregulation by oxidative stress in glioma cells. Biochim Biophys Acta 2001; 1512:335 - 344
- Hosoya K, Tomi M, Ohtsuki S, Takanaga H, Saeki S, et al. Enhancement of L-cystine transport activity and its relation to xCT gene induction at the blood-brain barrier by diethyl maleate treatment. J Pharmacol Exp Ther 2002; 302:225 - 231
- Kanai Y, Endou H. Heterodimeric amino acid transporters: molecular biology and pathological and pharmacological relevance. Curr Drug Metab 2001; 2:339 - 354
- Sato H, Tamba M, Okuno S, Sato K, Keino-Masu K, et al. et al. Distribution of cystine/glutamate exchange transporter, system x(c)-, in the mouse brain. J Neurosci 2002; 22:8028 - 8033
- Fernandez E, Jimenez-Vidal M, Calvo M, Zorzano A, Tebar F, et al. The structural and functional units of heteromeric amino acid transporters. The heavy subunit rBAT dictates oligomerization of the heteromeric amino acid transporters. J Biol Chem 2006; 281:26552 - 26561
- Wagner CA, Lang F, Broer S. Function and structure of heterodimeric amino acid transporters. Am J Physiol Cell Physiol 2001; 281:1077 - 1093
- Chillaron J, Roca R, Valencia A, Zorzano A, Palacin M. Heteromeric amino acid transporters: biochemistry, genetics and physiology. Am J Physiol Renal Physiol 2001; 281:995 - 1018
- Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, et al. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch 2004; 447:532 - 542
- Palacin M, Nunes V, Font-Llitjos M, Jimenez-Vidal M, Fort J, et al. The genetics of heteromeric amino acid transporters. Physiology (Bethesda) 2005; 20:112 - 124
- Bannai S, Christensen HN, Vadgama JV, Ellory JC, Englesberg E, et al. Amino acid transport systems. Nature 1984; 311:308
- Bannai S, Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol 1982; 112:265 - 272
- Christensen HN. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol Rev 1990; 70:43 - 77
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci 2002; 22:9134 - 9141
- Augustin H, Grosjean Y, Chen K, Sheng Q, Featherstone DE. Nonvesicular release of glutamate by glial xCT transporters suppresses glutamate receptor clustering in vivo. J Neurosci 2007; 27:111 - 123
- Piyankarage SC, Augustin H, Grosjean Y, Featherstone DE, Shippy SA. Hemolymph amino acid analysis of individual Drosophila larvae. Anal Chem 2008; 80:1201 - 1207
- Chung WJ, Lyons SA, Nelson GM, Hamza H, Gladson CL, et al. Inhibition of cystine uptake disrupts the growth of primary brain tumors. J Neurosci 2005; 25:7101 - 7110
- Savaskan NE, Heckel A, Hahnen E, Engelhorn T, Doerfler A, et al. Small interfering RNA-mediated xCT silencing in gliomas inhibits neurodegeneration and alleviates brain edema. Nat Med 2008; 14:629 - 632
- Sontheimer H. A role for glutamate in growth and invasion of primary brain tumors. J Neurochem 2008; 105:287 - 295
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, et al. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 2003; 6:743 - 749
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/glutamate exchange regulates metabotropic glutamate receptor presynaptic inhibition of excitatory transmission and vulnerability to cocaine seeking. J Neurosci 2005; 25:6389 - 6393
- Baker DA, Madayag A, Kristiansen LV, Meador-Woodruff JH, Haroutunian V, Raju I. Contribution of cystine-glutamate antiporters to the psychotomimetic effects of phencyclidine. Neuropsychopharmacology 33:1760 - 1772
- Featherstone DE, Shippy SA. Regulation of synaptic transmission by ambient extracellular glutamate. Neuroscientist 2007; 14:171 - 181
- Grosjean Y, Grillet M, Augustin H, Ferveur JF, Featherstone DE. A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat Neurosci 2008; 11:54 - 61
- Daniels RW, Gelfand MV, Collins CA, DiAntonio A. Visualizing glutamatergic cell bodies and synapses in Drosophila larval and adult CNS. J Comp Neurol 2008; 508:131 - 152
- Kolodziejczyk A, Sun X, Meinertzhagen IA, Nassel DR. Glutamate, GABA and acetyl-choline signaling components in the lamina of the Drosophila visual system. PLoS ONE 2008; 3:2110
- Devaud JM, Clouet-Redt C, Bockaert J, Grau Y, Parmentier ML. Widespread brain distribution of the Drosophila metabotropic glutamate receptor. Neuroreport 2008; 19:367 - 371
- Benzer S. From the gene to behavior. Jama 1971; 218:1015 - 1022
- Fergestad T, Olson L, Patel KP, Miller R, Palladino MJ, et al. Neuropathology in Drosophila mutants with increased seizure susceptibility. Genetics 2008; 178:947 - 956
- Ganetzky B, Wu CF. Indirect suppression involving behavioral mutants with altered nerve excitability in Drosophila melanogaster. Genetics 1982; 100:597 - 614
- Grigliatti TA, Hall L, Rosenbluth R, Suzuki DT. Temperature-sensitive mutations in Drosophila melanogaster XIV. A selection of immobile adults. Mol Gen Genet 1973; 120:107 - 114
- Homyk T Jr, Szidonya J, Suzuki DT. Behavioral mutants of Drosophila melanogaster III. Isolation and mapping of mutations by direct visual observations of behavioral phenotypes. Mol Gen Genet 1980; 177:553 - 565
- Kuebler D, Tanouye MA. Modifications of seizure susceptibility in Drosophila. J Neurophysiol 2000; 83:998 - 1009
- Gargano JW, Martin I, Bhandari P, Grotewiel MS. Rapid iterative negative geotaxis (RING): a new method for assessing age-related locomotor decline in Drosophila. Exp Gerontol 2005; 40:386 - 395
- Ferri SL, Bohm RA, Lincicome HE, Hall JC, Villella A. fruitless Gene products truncated of their male-like qualities promote neural and behavioral maleness in Drosophila if these proteins are produced in the right places at the right times. J Neurogenet 2008; 22:17 - 55