Abstract
Pathogenic bacteria, such as Vibrio cholerae, must be capable of adapting to diverse living conditions, especially when transitioning from life in environment reservoirs to life in a host. The ability to sense arrival at a site suitable for colonization or infection and responding with appropriate alterations in gene expression are crucial for a pathogen's success. Recently, we have shown that V. cholerae is able to recognize that it has reached its colonization site in the small intestine by sensing breakage of its flagellum as it penetrates the mucosal layer overlaying the intestinal epithelium. Flagellar loss results in the release of the anti-σ factor FlgM and subsequent activation of the alternative σ-factor FliA. FliA represses the quorum sensing-controlled transcriptional regulator, HapR, allowing increased expression of virulence factors such as Cholera Toxin (CT) and the Toxin Coregulated Pilus (TCP). In this way, the de-repression of virulence factor expression coincides with the arrival of bacteria at the site of infection at the intestinal mucosa. Our work reveals an interesting interplay between motility and quorum sensing signaling pathways to precisely time virulence gene expression during colonization.
Vibrio cholerae is the causative agent of the diarrheal disease, cholera, which can lead to death as a result of severe dehydration.Citation1 Virulence factors that mediate its pathogenesis include the cholera toxin, CT, and the Toxin Coregulated Pilus, TCP, the primary colonization factor. Both CT and TCP are regulated in response to cell density as well as other environmental cues.Citation2,Citation3 The process by which gene expression is altered in response to changes in cell density is known as quorum sensing.Citation4–Citation6 Each bacterium produces signaling molecules, termed autoinducers, that build up extraceljunlularly with increasing cell density, and these autoinducers are detected by cognate sensor kinase proteins, which transmit information downstream through a phosphorelay system. When the sensor kinase proteins are unbound by autoinducer at low cell density, the response regulator, LuxO, is phosphorylated, and it induces the expression of several small regulatory RNAs that repress expression of the transcriptional regulator, HapR. At high cell density, autoinducers bind to their cognate sensor kinases, causing a conformational change that results in reversed phosphate flow through the signaling cascade.Citation7–Citation10 LuxO is dephosphorylated, the small regulatory RNAs are no longer transcribed, and HapR is therefore expressed at high cell density. HapR mediates many downstream effects, among which is repression of virulence factorsCitation2 via repression of the transcriptional regulator, AphA, which is necessary for optimal expression of CT and TCP.Citation11 Hence, virulence factors are expressed at low cell density and repressed at high cell density as a result of regulation of HapR by the quorum sensing signaling pathway. V. cholerae also regulates virulence in response to environmental cues via the ToxRS and TcpPH regulatory complexes.Citation12 Expression of TcpPH requires AphA,Citation13 and thus both cell density and environmental sensory information are integrated at the level of HapR and AphA (). This allows the bacteria to adjust virulence factor production according to the specific environmental conditions they are in.
A large inoculum of V. cholerae is necessary for successful infection of a host due to the effects of a variety of hostile compounds, such as gastric acid and bile salts encountered by bacteria during passage through the upper digestive tract. Therefore, bacterial numbers are greatly reduced by the time they reach the small intestine. The drop in hapR expression, which accompanies the drop in bacterial numbers, is necessary for maximal expression of virulence factors.Citation14 While a decrease in autoinducer levels alone will trigger phosphorylation of LuxO and repression of HapR, it seems that bacterial cell density upon arrival in the small intestine could vary greatly from host to host. Since optimal virulence factor expression is only possible when hapR expression is repressed, it would be efficient for bacteria to be able to recognize a signal consistent across all hosts to alert them that they have reached an appropriate site for colonization, and to repress HapR production to facilitate the production of virulence factors. We have recently identified such a signal: flagellar breakage during mucosal penetration.Citation15 All V. cholerae must encounter and penetrate the mucosal layer prior to colonizing the small intestine. Our findings suggest that even though flagella are necessary for initial penetration of the mucosal layer, they are subsequently broken and are unnecessary for migration through the remainder of the mucosal layer. In addition, the anti-σ28 factor FlgM16 is secreted from the bacterial cells after flagellar loss, and this results in activation of FliA (σ28). FliA then represses HapR either directly or indirectly, and this process allows for high levels of CT and TCP expression (). Since V. cholerae must penetrate the mucosal layer just prior to colonization, this mechanism ensures that HapR will be repressed and virulence factors will be maximally expressed at this crucial time in the infectious cycle. The exact mechanism of V. cholerae flagellum-independent penetration of the mucosal layer is still not well understood. However, we have found that other bacteria, such as Escherichia coli, may employ the same mechanism to migrate through mucus (data not shown).
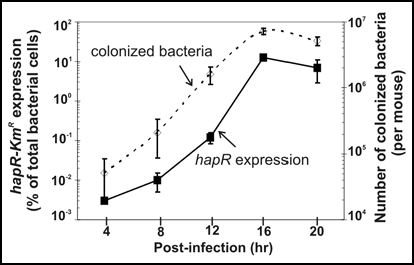
While we showed that HapR expression levels in various flagellar mutants fit our model in vitro, the most exciting data were from our mouse colonization model, which showed that the integration of quorum sensing and flagellar regulatory networks is truly in play in vivo during colonization. We used a hapR-kmR transcriptional fusionCitation17 to monitor HapR expression in the mouse () and showed that at an early time point after inoculation, when the bacterial cell density is presumably low, HapR levels are low in the wild-type strain. At the same time point, HapR levels are approximately 50-fold higher in the fliA mutant, since FliA is not present to repress HapR. This difference in HapR expression levels is actually important for pathogenesis, because the fliA mutant has a colonization defect beyond what is seen in other motility mutants. Later in the course of infection, HapR levels increase in both strains, consistent with increasing cell density and subsequent loss of small regulatory RNA repression of HapR via the quorum sensing signaling cascade. It is thought that HapR repression of virulence gene expression at high cell density allows for detachment and spread of the bacteria to new colonization sites within the same host or to the outside environment where they can encounter and infect a new host.Citation2
Our work helps to illuminate the complex and sophisticated crosstalk between motility and quorum sensing pathways that allows the successful pathogen V. cholerae to precisely time virulence gene expression during the course of infection.Citation18 Expression of virulence factors is crucial for effective colonization and infection, but repression of these same factors is necessary for detachment and dissemination, which are equally important parts of the infectious cycle.Citation19 In the case of V. cholerae, simultaneous low cell density and flagellar breakage signal penetration of the mucosal layer and arrival at the intestinal epithelial cells, and bacterial cells respond by repressing HapR and elaborating virulence factors. High cell density signals the end of the infectious cycle, and HapR repression of virulence factors allows exit from the intestine so that a new infectious cycle can begin.
Figures and Tables
Figure 1 Repression of HapR During Mucosal Penetration. Due to the harsh conditions of the upper digestive tract, V. cholerae reaches the small intestine in relatively low numbers. At low cell density, the quorum sensing response regulator, LuxO, is phosphorylated and represses HapR indirectly via small regulatory RNAs. As the bacteria penetrate the mucus covering the intestinal epithelial cells, their flagella break, FlgM is secreted, and FliA is activated, further repressing HapR. Since HapR represses virulence factor (CT and TCP) expression, these mechanisms of HapR repression during mucosal penetration serve to increase virulence gene expression.
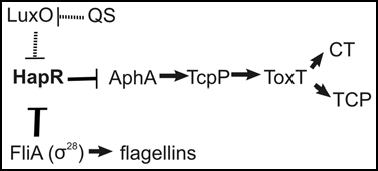
Figure 2 Quorum Sensing Regulation in vivo. Approximately 106 V. cholerae containing the hapR-Kmr chromosomal reporter were orally inoculated into infant CD-1 mice. At various time points, mouse intestines were homogenized, treated with or without kanamycin (500 µg/ml) for 10 min, and plated on LB plates. The number of colonized bacteria (dashed line) was calculated based on the number of colony-forming units (CFU) recovered from samples not treated with kanamycin. Expression of hapR (solid line) was defined as the percentage of Km-surviving cells out of total CFU. Results are means from experiments with three mice ± standard deviations.
Acknowledgements
This work is supported by NIH Grant R01AI072479 and NIH T32 Bacteriology training grant.
Addendum to:
References
- Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol Mol Biol Rev 1998; 62:1301 - 1314
- Zhu J, Miller MB, Vance RE, Dziejman M, Bassler BL, Mekalanos JJ. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci USA 2002; 99:3129 - 3134
- Peterson KM. Expression of Vibrio cholerae virulence genes in response to environmental signals. Curr Issues Intest Microbiol 2002; 3:29 - 38
- Whitehead NA, Barnard AM, Slater H, Simpson NJ, Salmond GP. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol Rev 2001; 25:365 - 404
- Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 2005; 21:319 - 346
- Fuqua WC, Winans SC, Greenberg EP. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J Bacteriol 1994; 176:269 - 275
- Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae. Cell 2002; 110:303 - 314
- Lenz DH, Mok KC, Lilley BN, Kulkarni RV, Wingreen NS, Bassler BL. The small RNA chaperone Hfq and multiple small RNAs control quorum sensing in Vibrio harveyi and Vibrio cholerae. Cell 2004; 118:69 - 82
- Higgins DA, Pomianek ME, Kraml CM, Taylor RK, Semmelhack MF, Bassler BL. The major Vibrio cholerae autoinducer and its role in virulence factor production. Nature 2007; 450:883 - 886
- Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae. Developmental cell 2003; 5:647 - 656
- Kovacikova G, Skorupski K. Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter. Mol Microbiol 2002; 46:1135 - 1147
- Matson JS, Withey JH, DiRita VJ. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun 2007; 75:5542 - 5549
- Skorupski K, Taylor RK. A new level in the Vibrio cholerae ToxR virulence cascade: AphA is required for transcriptional activation of the tcpPH operon. Mol Microbiol 1999; 31:763 - 771
- Svenningsen SL, Waters CM, Bassler BL. A negative feedback loop involving small RNAs accelerates Vibrio cholerae's transition out of quorum-sensing mode. Genes Dev 2008; 22:226 - 238
- Liu Z, Miyashiro T, Tsou A, Hsiao A, Goulian M, Zhu J. Mucosal penetration primes Vibrio cholerae for host colonization by repressing quorum sensing. Proc Natl Acad Sci USA 2008; 105:9769 - 9774
- Hughes KT, Gillen KL, Semon MJ, Karlinsey JE. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science 1993; 262:1277 - 1280
- Liu Z, Stirling FR, Zhu J. Temporal quorum-sensing induction regulates Vibrio cholerae biofilm architecture. Infect Immun 2007; 75:122 - 126
- Hsiao A, Liu Z, Joelsson A, Zhu J. Vibrio cholerae virulence regulator-coordinated evasion of host immunity. Proc Natl Acad Sci USA 2006; 103:14542 - 14547
- Nielsen AT, Dolganov NA, Otto G, Miller MC, Wu CY, Schoolnik GK. RpoS controls the Vibrio cholerae mucosal escape response. PLoS Pathog 2006; 2:109