Abstract
Microtubules are cytoskeletal structures in the cytoplasm of eukaryotic cells, and their highly dynamic properties are essential to perform a wide variety of vital functions in cells. Microtubule growth proceeds through the endwise addition of nucleotide-bound tubulin molecules. It has largely been assumed that only tubulin dimers can incorporate into microtubules, and that the chemical state of the nucleotide is crucial for the incorporation. Recent observations reveal that both tubulin dimers and oligomers can add to microtubule ends and that the chemical state of the nucleotide is not decisive for tubulin addition. Together with structural studies of tubulin, these results show tubulin assembly polymorphism, which could play a crucial role in microtubule-dependent cellular functions.
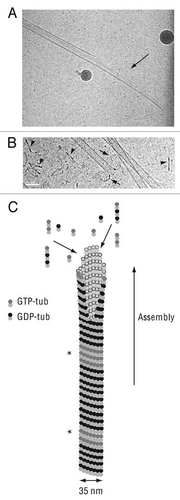
Microtubules are key components of the eukaryotic cell cytoskeleton and they have important roles in many cellular processes such as intracellular transport, cell motility, cell morphogenesis, meiosis and mitosis. The basic structural unit of microtubules is the αβ-tubulin heterodimer. Tubulin heterodimers bind head to tail to form protofilaments. In the general case, 13 protofilaments associate to give rise to a cylindrical microtubule, in which each tubulin molecule has lateral and longitudinal interactions with its neighbours in the polymer wall. Microtubules can switch between growing and shortening phases, a phenomenon known as dynamic instability.Citation1,Citation2 During the growing phase, tubulin molecules are added at the end of polymers. Microtubules shorten through tubulin loss from their tips. In cells, dynamic instability is highly regulated by microtubule associated proteins (reviewed in ref. Citation3 and Citation4). However, this dynamic behavior is an intrinsic property of microtubules themselves, observable also in microtubules assembled from purified tubulin suspensions. Actually, our knowledge of how microtubules grow and shrink is principally based on in vitro studies performed with pure tubulin ( and B).
α- and β-tubulin monomers carry nucleotide binding sites in their N-terminal domain. The α-subunit is constitutively associated to a non-exchangeable GTP, buried at the monomermonomer interface. In contrast, the β-tubulin subunit comprises a binding site exposed at the protein surface and at which the nucleotide is exchangeable (E-site). Following the addition at microtubule end of a tubulin dimer containing GTP on E-site (GTP-tub), the E-site GTP is hydrolyzed and the newly generated GDP becomes non-exchangeable. As a result the microtubule wall is mainly composed of tubulin subunits containing GDP on E-site (GDP-tub).
A recent study has shown that, irrespective of their nucleotide binding state (GTP, GDP or a slowly hydrolysable analogue of GTP, GMPCPP), the majority of tubulin dimers in solution form small oligomers.Citation5 Moreover, GDP-tub oligomers are released from shortening microtubulesCitation6,Citation7 (). Thus, a simple chemical system containing only tubulin and GTP in appropriate conditions of buffer and temperature will ultimately be composed of multiple forms of tubulin molecules, GTP-tub and GDP-tub dimers and oligomers and microtubules. This review focuses on how these various tubulin subunits and polymer intermediates contribute to, and modulate, microtubule growth and dynamics.
Do Tubulin Oligomers Participate to Microtubule Elongation?
In recent experiments, individual microtubule elongation has been tracked at molecular scale. Two sets of experiments using optical tweezers showed that the microtubule length increases by step during polymer elongation.Citation7,Citation8 However length increment measurements were not similar in both studies and these observations led to different conclusions. One report shows that small tubulin oligomers (20–30 nm i.e., 2 to 4 associated tubulin dimers) are able to add directly to microtubule tipsCitation8 whereas the other study indicates that only tubulin dimers (8 nm) are incorporated.Citation7 Electron microscopy experiments suggest also that microtubules can elongate through tubulin oligomers addition.Citation5
Previous electron microscopy studies have shown that growing microtubule ends form intermediate sheet structures that subsequently close into tubesCitation9 (). An implication of this finding is that tubulin molecules may add either at protofilament tips or within sheets by lateral interactions with tubulin subunits forming the sheet edge. Tubulin addition at the end of protofilament directly increases microtubule length and is detected by optical microscopy measurements. In contrast, the incorporation of tubulin molecules in the sheet does not participate to immediate microtubule length increaseCitation5 and is not revealed using optical microscopy. The incorporation of tubulin as dimers or oligomers is therefore still a matter of debate.
GTP-Tubulin Remnants in Microtubules
Microtubules are able to polymerize in the presence of GMPCPP (GMPCPP-microtubules).Citation10 Structural differences have been detected in the lattice of GMPCPP-microtubules and of microtubules assembled from GTP, thereby composed of GDP-tub (GDP-microtubules).Citation11 Recently, an antibody was selected that recognizes specifically GMPCPP-microtubules but not GDP-microtubules.Citation12 In cells, the antibody stains growing microtubule ends, and also short fragments in the middle of microtubules. The authors suggest that GTP-tubulin remnants could be present in microtubule lattice, indicating that GTP hydrolysis is sometimes incomplete during polymerization.Citation12 Interestingly, the position of these internal sites seems to correlate in cells with transitions from microtubule shrinkage to growth. GTP-remnants would then have a structural state more stable than the whole microtubule lattice. This new state remains to be structurally characterized. This study shows that GTP-tub can also be included in microtubules without GTP hydrolysis step, providing intrinsic chemical variability to microtubule lattice.
GDP-tub is Directly Incorporated in Microtubules
The possibility of a direct incorporation of GDP-tub into growing microtubules has been raised at early stages of microtubule research, and has remained a matter of controversy. Essentially based on turbidimetry measurements, an increase of polymerized tubulin was reported in suspension containing tubulin and GTP in the presence of GDP-tub.Citation13–Citation15 These results suggested that GDP-tub was directly assembled into microtubules, although GDP-tub incorporation was not directly measured. Alternatively, other studies demonstrated that the addition of GDP-tub to microtubule suspension does not participate significantly to microtubule elongation.Citation16–Citation19 The effect of GDP-tub on microtubule dynamics has been studied and results also exhibited discrepancies. Reports have shown that GDP-tub stabilizes microtubulesCitation17,Citation18 while in another study the principal effect of GDP-tub is an increase of catastrophe and rescue frequencies, with the occurrence of growth and shortening irregularities.Citation20
Recently, the question of direct GDP-tub incorporation in microtubules was re-addressed using a minimal tubulin assembly system composed of nucleotide bound tubulin dimers, in the absence of excess free nucleotide.Citation13,Citation21–Citation24 Such a system has two main advantages: (1) the proportion of GTP-tub and GDP-tub added in solution can be controlled at will and (2) measurements of direct incorporation of both nucleotide-bound tubulin dimers can be performed.Citation24 Results demonstrate that substantial amounts of GDP-tub can be directly incorporated into growing microtubules. Moreover microtubules assembled from GTP-tub and GDP-tub mixtures display modified dynamic behavior. Video-microscopy experiments performed with microtubules nucleated on centrosomes demonstrated that growth and shrinkage rates decreased by half in the presence of 30% GDP-tub in the starting mix. The decrease of the shrinkage rate was of particular interest because microtubule shrinkage seems to arise from a zero order reaction, governed by intrinsic factors reflecting the structural state of the microtubule lattice.
Previous works have established that GTP hydrolysis is not necessary for tubulin assembly, but rather for microtubule disassembly.Citation10,Citation25 When tubulin is assembled in the presence of GMPCPP, resulting GMPCPP-microtubules are stable compared with GDP-microtubules.Citation10 This strongly suggests that much of the energy released by GTP hydrolysis is stored in the microtubule lattice and released during microtubule disassembly. The direct incorporation of GDP-tub into microtubule lattice is obviously not followed by hydrolysis of the bound nucleotide. Therefore it could be suggested that GDP may function as a natural non-hydrolyzable analog of GTP, with resulting impairment of the disassembly properties of microtubules. Detectable modifications have been found in the lattice organization of GMPCPP-microtubules compared to GDP-microtubules.Citation11 No structural modification was observed in microtubules that incorporated directly GDP-tub. It is possible that lattice modification would be blurred by the co-incorporation of GTP-tub and GDP-tub, compared to the homogeneity of GMPCPP-microtubules.
Structural Changes in Tubulin
Structural studies of tubulin heterodimer in various nucleotide and polymerization states have been reported in the past few years. They have led to two challenging models for the relationship between GTP and conformational changes of tubulin molecule. A first set of reports indicate that GTP-tub has a straight conformation, α and β tubulin subunits being aligned, while GDP-tub adopts a more bent conformation, with a kink between both tubulin subunits.Citation26,Citation27 These results lead to a model in which curved GDP-tub would then be unable to form the lateral contacts necessary to fit the microtubule lattice, therefore would be unable to participate to microtubule elongation. Following nucleotide exchange, GTP-tub would straighten and lateral contacts between adjacent tubulin molecules could become possible.Citation26,Citation27 More recently, another study suggests that free GTP-tub and GDP-tub in solution may be similarly bent. Major structural rearrangements of tubulin would not occur in response to GTP binding. Tubulin would straighten only after incorporation into growing microtubules, as a consequence of the straightening of protofilaments during tube closure.Citation28 GTP only tunes the strength of contacts in the microtubule lattice. This model is compatible with a direct incorporation of GDP-tub within microtubule lattice, together with GTP-tub. Actually GDP-tub alone is not able to assemble into microtubules.Citation13,Citation19,Citation24
These models only consider the addition of tubulin dimers into microtubule lattice. Results reported above suggest that tubulin oligomers are also able to add either into terminal sheet at microtubule tip or directly at the end of protofilaments. Within these oligomers, it seems likely that a proportion of tubulin-bound GTP is hydrolyzed so that oligomers are composed of both GTP-tub and GDP-tub. Such addition of macromolecular assembly of tubulin dimers remains to be structurally investigated.
Conclusions
Multiple macromolecular tubulin assemblies can be formed from purified tubulin molecules, depending on nucleotide bound and oligomerization states. Following the classical textbook model, microtubules elongate exclusively by addition of GTP-tub. In contrast with this model, it has been shown that tubulin dimers as well as these structural intermediates may be used in microtubule elongation that therefore shows a high degree of plasticity. The recent reports of GDP-tub incorporation and GTP-tub remnants suggest that the tubulin subunits constituting the microtubule wall are in different structural states according to their initial nucleotide bound state, with resulting variations in intrinsic microtubule dynamic properties (). These findings reveal a novel form of microtubule “structural plasticity”.Citation29 Moreover, the polymorphism of microtubule assembly could reflect mechanisms that tune the interactions between microtubules and cellular factors.Citation30 To go further, we will need probes that recognize different structural states of microtubules in cells. An antibody is already available that recognizes specific structural states.Citation12 May be, other binding factors, either antibodies or drugs or proteins, will prove to be useful reporters of the microtubule structural status, which could facilitate in vivo investigation of microtubule structural plasticity.
Abbreviations
| GTP-tub | = | tubulin associated to GTP on exchangeable site |
| GDP-tub | = | tubulin associated to GDP on exchangeable site |
| GMPCPP | = | guanylyl-(α,β)-methylene-diphosphonate |
| GMPCPP-microtubules | = | microtubules assembled in the presence of GMPCPP |
| GDP-microtubules | = | microtubules assembled in the presence of GTP, followed by GTP hydrolysis in the polymer wall |
Figures and Tables
Figure 1 Microtubule assembly. (A) Cryo-electron microscopy image of a growing microtubule end showing an opened sheet (arrow). (B) Cryoelectron microscopy image of a shrinking microtubule end showing protofilaments peeling into ring-like structures (arrows). Numerous tubulin oligomers coming from microtubule disassembly are visible in the background (arrowheads). Scale bar, 50 nm in both parts. (C) Schematic representation of a growing microtubule. The end displays a sheet-like conformation which later close into a tube. GTP-tub dimers, GDP-dimers and tubulin oligomers participate to microtubule elongation at the end of the polymer. GTP-tub, α-tubulin and β-tubulin associated to GTP; GDP-tub, α-tubulin and β-tubulin associated to GDP; *indicates GTP-remnants integrated in the microtubule wall. Cryo-electron microscopy images are courtesy of Dr. I. Arnal, UMR 6026, Rennes, France.
Acknowledgements
I thank Dr. I. Arnal and Dr. D. Job for critical reading of the manuscript and Dr. I. Arnal for and B.
References
- Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol 1997; 13:83 - 117
- Valiron O, Caudron N, Job D. Microtubule dynamics. Cell Mol Life Sci 2001; 58:2069 - 2084
- Amos LA, Schlieper D. Microtubules and maps. Adv Protein Chem 2005; 71:257 - 298
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev Mol Cell Biol 2008; 9:309 - 322
- Mozziconacci J, Sandblad L, Wachsmuth M, Brunner D, Karsenti E. Tubulin dimers oligomerize before their incorporation into microtubules. PLoS ONE 2008; 3:3821
- Mandelkow EM, Mandelkow E, Milligan RA. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol 1991; 114:977 - 991
- Schek HT, Gardner MK, Cheng J, Odde DJ, Hunt AJ. Microtubule assembly dynamics at the nanoscale. Curr Biol 2007; 17:1445 - 1455
- Kerssemakers JW, Munteanu EL, Laan L, Noetzel TL, Janson ME, Dogterom M. Assembly dynamics of microtubules at molecular resolution. Nature 2006; 442:709 - 712
- Chrétien D, Fuller SD, Karsenti E. Structure of growing microtubule ends: two-dimensional sheets close into tubes at variable rates. J Cell Biol 1995; 129:1311 - 1328
- Hyman AA, Salser S, Drechsel DN, Unwin N, Mitchison TJ. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol Biol Cell 1992; 3:1155 - 1167
- Hyman AA, Chrétien D, Arnal I, Wade RH. Structural changes accompanying GTP hydrolysis in microtubules: information from a slowly hydrolyzable analogue guanylyl-(α,β)-methylene-diphosphonate. J Cell Biol 1995; 128:117 - 125
- Dimitrov A, Quesnoit M, Moutel S, Cantaloube I, Poüs C, Perez F. Detection of GTP-tubulin conformation in vivo reveals a role for GTP remnants in microtubules rescues. Science 2008; 322:1353 - 1356
- Carlier MF, Pantaloni D. Kinetic analysis of cooperativity in tubulin polymerization in the presence of guanosine di- or triphosphate nucleotides. Biochemistry 1978; 17:1908 - 1915
- Hamel E, Batra JK, Lin CM. Direct incorporation of guanine 5′-diphosphate into microtubules without guanosine 5′-triphosphate hydrolysis. Biochemistry 1986; 25:7054 - 7062
- Lin CL, Hamel E. Interrelationships of tubulin-GDP and tubulin-GTP in microtubule assembly. Biochemistry 1987; 26:7173 - 7182
- Jameson L, Caplow M. Effect of guanosine diphosphate on microtubule assembly and stability. J Biol Chem 1980; 255:2284 - 2292
- Zackroff RV, Weisenberg RC, Deery WJ. Equilibrium and kinetic analysis of microtubule assembly in the presence of guanosine diphosphate. J Mol Biol 1980; 139:641 - 677
- Caplow M, Reid R. Direct elongation model for microtubule GTP hydrolysis. Proc Natl Acad Sci USA 1985; 82:3267 - 3271
- Bayley PM, Butler FMM, Manser EJ. Control of nucleation in microtubule self-assembly. FEBS Lett 1986; 205:230 - 234
- Vandecandelaere A, Martin SR, Bayley PM. Regulation of microtubule dynamic instability by tubulin-GDP. Biochemistry 1995; 34:1332 - 1343
- O'Brien ET, Erickson HP. Assembly of pure tubulin in the absence of free GTP: effect of magnesium, glycerol, ATP and the nonhydrolyzable GTP analogues. Biochemistry 1989; 28:1413 - 1422
- Carlier MF, Didry D, Pantaloni D. Hydrolysis of GTP associated with the formation of tubulin oligomers is involved in microtubule nucleation. Biophys J 1997; 73:418 - 427
- Caudron N, Valiron O, Usson Y, Valiron P, Job D. A reassessment of the factors affecting microtubule assembly and disassembly in vitro. J Mol Biol 2000; 297:211 - 220
- Valiron O, Arnal I, Caudron N, Job D. GDP-tubulin incorporation into growing microtubules modulates polymer stability. J Biol Chem 2010; 285:17507 - 17513
- Caplow M, Ruhlen RL, Shanks J. The free energy for hydrolysis of a microtubule-bound nucleotide triphosphate is near zero: all the free energy for hydrolysis is stored in the microtubule lattice. J Cell Biol 1994; 127:779 - 788
- Müller-Reichert T, Chrétien D, Severin F, Hyman AA. Structural changes at microtubule ends accompanying GTP hydrolysis: information from a slowly hydrolyzable analogue of GTP, guanylyl-(alpha, beta)methylenediphosphonate. Proc Natl Acad Sci USA 1998; 95:3661 - 3666
- Wang HW, Nogales E. Nucleotide-dependent bending flexibility of tubulin regulates microtubule assembly. Nature 2005; 435:911 - 915
- Rice LM, Montabana EA, Agard DA. The lattice as allosteric effector: structural studies of α-, β- and γ-tubulin clarify the role of GTP in microtubule assembly. Proc Natl Acad Sci USA 2008; 105:5378 - 5383
- Kueh HY, Mitchison TJ. Structural plasticity in actin and tubulin polymer dynamics. Science 2009; 325:960 - 963
- Nogales E, Wang HW. Structural mechanisms underlying nucleotide-dependent self-assembly of tubulin and its relative. Curr Opin Struct Biol 2006; 16:1 - 9