Abstract
Bees, wasps and ants navigate successfully between feeding sites and their nest, despite the small size of their brains which contain less than a million neurons. A long history of studies examining the role of visual memories in homing behavior show that insects can localise a goal by finding a close match between a memorized view at the goal location and their current view (“snapshot matching”). However, the concept of static snapshot matching might not explain all aspects of homing behavior, as honeybees are able to use landmarks that are statically camouflaged. In this case the landmarks are only detectable by relative motion cues between the landmark and the background, which the bees generate when they perform characteristic flight manoeuvres close to the landmarks. The bees’ navigation performance can be explained by a matching scheme based on optic flow amplitudes (“dynamic snapshot matching”). In this article, I will discuss the concept of dynamic snapshot matching in the light of previous literature.
Goal-Seeking in Insects
Insects, with their miniature brains, have evolved a simple strategy to find both their nests and profitable food sources. They approach them by “snapshot matching”, i.e., finding a close match between the current view and a memorized view of the goal location (reviewed in ref. Citation1 and Citation2). How is the relevant information encoded in a snapshot? There are currently two main views: (1) Insects may extract the relevant visual features, such as the contours of prominent objects which define the goal location.Citation3 In addition to retinal size and the position of these objects, honeybees also use color and distance cues as features to localize a food source.Citation3–Citation8 Currently, it is still unclear how many and what features are stored in the retinotopic snapshot. (2) A fairly new idea, which does not involve the extraction of certain features and object identification, is the “global image matching” method.Citation9 This means that the insects memorize a raw panoramic image at the goal location. The image implicitly contains all important static visual features of the goal location. The insects then get back to their goal location by moving in a way that increases the similarity of the current view with the memorized image. Recent model implementations on robotic platforms show that this method works.Citation9–Citation14 Even for view-based homing in natural environments, Zeil and colleagues showed that panoramic image similarities can be used.Citation9,Citation15 More recently, the behavior of ants and crickets in goal finding tasks could be explained by “global image matching”.Citation16,Citation17
Dynamic Snapshots
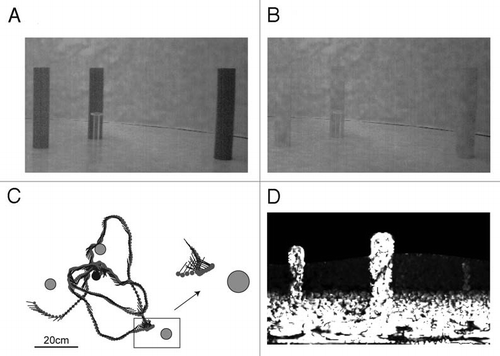
Within the insect navigation literature, snapshot matching is presently often pictured as a static process, which involves three steps: stopping, comparing the current view with the memorized view at the goal, and then moving to increase the similarity between the views. However, our study shows that static snapshot matching might not explain all aspects of homing behavior as honeybees are able to use landmarks that are statically camouflaged.Citation18 Honeybees, trained to locate an inconspicuous feeder surrounded by three high contrast landmarks (), were tested either with one landmark removed or with three landmarks with the same contrast and texture as the background (). Removing a landmark affected the search performance of the honeybees. Surprisingly, the navigational performance was not impaired when the landmarks were camouflaged.Citation18 In this case the landmarks could not be detected by their contrast and texture, and if the bees would had memorized a static image of the scene they would not have been able to find the goal. These landmarks only become visible when the bee moves in a characteristic way (see inset).
We found that honeybees perform scanning movements in the vicinity of the landmarks.Citation18 Through these sideways maneuvers in front of the landmarks, they become visible by relative motion. Furthermore, they employ a flight style that facilitates depth perception from motion parallax. The bees' trajectories consist of straight flight segments combined with rapid turns.Citation18,Citation19 Between turns, gaze stabilization leads to a behavioral elimination of rotational components from the optical flow pattern,Citation19,Citation20 making it easier to use the remaining translational optic flow for homing, as it contains range information. This is because images of close objects move faster across the retina than those of more distant objects. In our experiments with landmarks that had the same texture as the background this helped detection and possibly also estimation of the distance to landmarks as they ‘pop out’ when the animals move in front of them. In other behavioral tasks, motion parallax is used as a cue, e.g., for shape discrimination or distance estimation.Citation21,Citation22
But what does a snapshot look like, and what information is stored? By means of model simulations, we made a first attempt to answer this question. Global image matching cannot explain the search behavior with statically camouflaged landmarks. Taking the honeybees movement into account, we simulated the motion pattern on the bees' eyes during pure translational movements at the goal location (). By memorizing this optic flow snapshot and comparing it with the optic flow fields at other locations in the flight arena, the honeybees could navigate even with camouflaged landmarks.Citation18 Our study emphasises that snapshot matching should be considered a dynamic rather than a purely static process. Taking into account how the insects interact with their environment helped us to identify possible mechanisms of navigation.
Contents and Extent of Retinotopic Snapshots
Neither a static nor a dynamic snapshot can explain all the results obtained from navigation experiments. When trained with different colored landmarks, honeybees use also color cues.Citation4 Perhaps a snapshot consists of landmark boundaries, which can be detected by luminance, color or motion contrast. You might also think of a combination of optic flow and texture information in an extended snapshot scheme.
The selection and weighting of static or dynamic information might vary among different species and depend on the kind of locomotion the insect performs. Walking insects (e.g., ants) can more easily stay motionless relative to their surroundings (an advantage for static image matching) whereas flying insects (e.g., bees) can fly sideways (an advantage if using motion parallax information). There might also exist species-dependent differences in whether the insect memorizes a snapshot of the full field of view and whether all regions of the visual field are weighted equally. Ants store a snapshot while fixating a landmark with their frontal field of view, which extends at least 120° into the periphery.Citation23 When trained with several landmarks surrounding the nest or food source, they focus on their frontal field of view and employ a sequential matching strategy of single landmarks.Citation24,Citation25 If the landmark array is enlarged without changing the size of the landmarks, the ants do not search where the retinal position of the landmarks fits but where the retinal size of a single landmark fits.Citation25–Citation27 In contrast, bees tested under similar conditions seem to match the retinal positions of all landmarks, if there is a conflict with the retinal size of the landmarks.Citation3 But although the snapshot is centred on one landmark in ants, it was concluded that they identify landmarks with the aid of the background pattern, indicating that the panorama is somehow included in their representation and might help to enhance the reliability of the snapshot recall.Citation27 An experiment, in which different species are tested with the same behavioral task, might provide insights into the relevance and weighting of different navigational mechanisms and the contents and size of snapshot memories.
A Single Snapshot? Where and When?
Despite a series of studies on snapshot matching in insects, there remain some crucial questions to be answered. Today, it is still unclear how many snapshots the insects memorize and where they take these snapshots. It might be that there is no universal answer to these questions. How snapshots are used for navigation probably depends on the species, and even on the task or the learning state of the insect. Nevertheless, differences can teach us something about the underlying navigational mechanisms and their constraints. Although agents can navigate successfully to a goal location with the aid of one snapshot close to the goal, e.g.Citation12 there is evidence that ants, for example, memorize several snapshots and use them for returning to the goal location.Citation28,Citation29 The current idea is that snapshots are taken during learning walks in ants and during learning flights in bees and wasps.Citation30–Citation33 These learning phases have a common feature: when leaving the nest or a newly discovered food source, the insects turn back and perform distinct maneuvers. The learning walks of ants include stopping phases, in which they fixate nearby landmarks (wood antsCitation24) or the goal itself (Namibian desert antsCitation33) and approach the target for a short time. The acquisition of memories is thought to take place during these clear-cut events of the learning walks. Wasps and honeybees are thought to memorize snapshots while fixating the goal at the end of arcs (inspection points), when they turn back and look at the feeder during their learning flights.Citation30 Assuming that honeybees would indeed memorize an optic flow snapshot, it could take place during the translatory phases of flight when they face their goal.Citation19
These straight segments of the learning flights provide them with spatial information, e.g., the distance to the landmarks, which fits to the long standing interpretation that learning flights help the insects to identify close landmarks and learn their spatial relationship.Citation31,Citation32,Citation34–Citation36 If insects memorize snapshots during these learning maneuvers, they should follow a somewhat similar trajectory in their next return to the goal. But instead the similarity of viewing directions and flight paths between learning and return flights seems to differ between species and is less striking in honeybees.Citation30,Citation32,Citation37,Citation38 On the other hand, the insects might replicate some of the relevant movement sequences without following the same paths. The motion dynamics of learning and return flights are similar in beesCitation19 and wasps (Boeddeker personal communication). The functional connection between learning walks or flights and returns still has to be understood. Analysing the detailed movements of the insects during navigation should give further insights. In a recent study, Lent and colleagues analyzed the movements of ants during the return to a goal in detail.Citation39 These wood ants perform saccadic-like body turns to match the learned visual features. The turns depend on the difference between the desired and current retinal positions of a visual feature, and they are also used to correct errors after the ants have drifted off the route. Although this provides us with an idea how snapshots are used to guide the ants' path it is yet unclear, how this connects to their learning walks. Do insects memorize all possible snapshots during their learning maneuvers? How are these snapshots used to structure the return? Learning maneuvers only occur in the early phase of foraging and even the return paths change over time.Citation6,Citation40 It might well be that several snapshots are somehow integrated into one spatial representation of the goal environment over time, allowing the insect to return reliably but at the same time to navigate flexibly to the goal location. I suggest tackling these questions using comparative studies and by analysing the insects' behavior in detail (e.g. ref. Citation38, Citation39, Citation41, reviewed in ref. Citation42). Identifying differences in the navigational strategies depending on the species, the task, the complexity of the visual environment, and the learning state of the insect, will help us understand the relevance and the constraints of the underlying navigational mechanisms.
Figures and Tables
Figure 1 In our study honeybees were trained to visit a perspex feeder surrounded by three landmarks (diameter of 5 cm, height of 25 cm) placed at different distances (10, 20, 40 cm) from the feeder in a circular flight arena (diameter of 195 cm; height of 50 cm).Citation18 (A) During training the landmarks were covered with a homogeneous texture providing luminance contrast to the background. (B) After being trained with three homogenous textured landmarks, honeybees were tested individually with landmarks that had the same random dot texture as the background of the arena. (C) The top view shows an example flight trajectory of a honeybee. Approach flights to the feeder (black circle) surrounded by three landmarks (grey circles) were recorded with three high-speed cameras at 125 fps. The position of the bee is indicated by grey circles at each 32 ms interval; straight lines indicate the orientation of the long axis of the bee. The inset shows a sideways-directed flight manoeuvre of a honeybee close to the landmark (two times magnified; Reproduced from ref. Citation18). (D) shows an optic flow snapshot calculated at the feeder position. White indicates high optic flow amplitudes. The landmarks “pop out” of the background although they have the same texture as the background (B). From a 3D model of the arena, panoramic images were rendered at 1°/pixel resolution. Optic flow fields were generated by simulated translations of 2 mm in the flight arena, obtaining four images at (x ± 1 mm, y), (x, y ± 1 mm). The optic flow for these movements was computed using a modified version of the Lucas-Kanade algorithm. To generate flow amplitudes independent of the direction of motion, flow fields for the two steps (in x and y) were squared and summed.Citation18 The optic flow snapshot implicitly contains information about the depth structure of the scene, as closer objects move faster and create a higher optic flow amplitude. This can be seen by comparing the near landmark in the centre of the picture to the middle far (left) and the far (right) landmark.
Acknowledgements
I thank Norbert Boeddeker, Martin Egelhaaf and Nicole Carey for their helpful comments on the manuscript and Wolfgang Stürzl for providing the “optic flow snapshot” image. Financial support came from the DFG.
References
- Collett TS, Graham P, Harris RA, de Ibarra NH. Navigational memories in ants and bees: Memory retrieval when selecting and following routes. Adv Study Behav 2006; 36:123 - 172
- Zeil J, Boeddeker N, Stürzl W. Floreano D, et al. Visual homing in insects and robots. Flying insects and robots 2009; New York, NY Springer 87 - 100
- Cartwright BA, Collett TS. Landmark learning in bees—experiments and models. J Comp Physiol 1983; 151:521 - 543
- Cheng K, Collett TS, Wehner R. Honeybees learn the colors of landmarks. J Comp Physiol A 1986; 159:69 - 73
- Cheng K, Collett TS, Pickhard A, Wehner R. The use of visual landmarks by honeybees—bees weight landmarks according to their distance from the goal. J Comp Physiol A 1987; 161:469 - 475
- Fry SN, Wehner R. Look and turn: landmark-based goal navigation in honey bees. J Exp Biol 2005; 208:3945 - 3955
- Cartwright BA, Collett TS. How honeybees know their distance from a near-by visual landmark. J Exp Biol 1979; 82:367 - 372
- Lehrer M, Collett TS. Approaching and departing bees learn different cues to the distance of a landmark. J Comp Physiol A 1994; 175:171 - 177
- Zeil J, Hofmann MI, Chahl JS. Catchment areas of panoramic snapshots in outdoor scenes. J Opt Soc Am A 2003; 20:450 - 469
- Franz MO, Schölkopf B, Mallot HA, Bülthoff H. Where did I take that snapshot? Scene-based homing by image matching. Biol Cybern 1998; 79:191 - 202
- Stürzl W, Mallot HA. Efficient visual homing based on Fourier transformed panoramic images. Robot Auton Syst 2006; 54:300 - 313
- Vardy A, Möller R. Biologically plausible visual homing methods based on optical flow techniques. Connect Sci 2005; 17:47 - 89
- Möller R, Vardy A. Local visual homing by matched-filter descent in image distances. Biol Cybern 2006; 95:413 - 430
- Möller R. Local visual homing by warping of two-dimensional images. Robot Auton Syst 2009; 57:87 - 101
- Stürzl W, Zeil J. Depth, contrast and view-based homing in outdoor scenes. Biol Cybern 2007; 96:519 - 531
- Wystrach A, Beugnon G. Ants learn geometry and features. Curr Biol 2009; 19:61 - 66
- Mangan M, Webb B. Modelling place memory in crickets. Biol Cybern 2009; 101:307 - 323
- Dittmar L, Stürzl W, Baird E, Boeddeker N, Egelhaaf M. Goal seeking in honeybees: matching of optic flow snapshots?. J Exp Biol 2010; 213:2913 - 2923
- Boeddeker N, Dittmar L, Stürzl W, Egelhaaf M. The fine structure of honeybee head and body movements in a homing task. Proc Biol Sci 2010; 277:1899 - 1906
- Boeddeker N, Hemmi JM. Visual gaze control during peering flight manoeuvres in honeybees. Proc Biol Sci 2010; 277:1209 - 1217
- Lehrer M, Campan R. Generalization of convex shapes by bees: what are shapes made of?. J Exp Biol 2005; 208:3233 - 3247
- Srinivasan MV, Zhang SW. Visual motor computations in insects. Annu Rev Neurosci 2004; 27:679 - 696
- Durier V, Graham P, Collett TS. Snapshot memories and landmark guidance in wood ants. Curr Biol 2003; 13:1614 - 1618
- Nicholson DJ, Judd SPD, Cartwright BA, Collett TS. Learning walks and landmark guidance in wood ants (Formica rufa). J Exp Biol 1999; 202:1831 - 1838
- Narendra A, Si A, Sulikowski D, Cheng K. Learning, retention and coding of nest-associated visual cues by the Australian desert ant, Melophorus bagoti. Behav Ecol Sociobiol 2007; 61:1543 - 1553
- Wehner R, Räber F. Visual spatial memory in desert ants, Cataglyphis bicolor (Hymenoptera, Formicidae). Experientia 1979; 35:1569 - 1571
- Graham P, Durier V, Collett TS. The binding and recall of snapshot memories in wood ants (Formica rufa L.). J Exp Biol 2004; 207:393 - 398
- Judd SPD, Collett TS. Multiple stored views and landmark guidance in ants. Nature 1998; 392:710 - 714
- Harris RA, Graham P, Collett TS. Visual cues for the retrieval of landmark memories by navigating wood ants. Curr Biol 2007; 17:93 - 102
- Collett TS, Lehrer M. Looking and learning—a spatial pattern in the orientation flight of the wasp vespulavulgaris. P Roy Soc Lond B Biol 1993; 252:129 - 134
- Zeil J. Orientation flights of solitary wasps (Cerceris, Sphecidae, Hymenoptera): I. Description of flight. J Comp Physiol A 1993; 172:189 - 205
- Zeil J. Orientation flights of solitary wasps (Cerceris, Sphecidae, Hymenoptera): II. Similarities between orientation and return flights and the use of motion parallax. J Comp Physiol A 1993; 172:207 - 222
- Müller M, Wehner R. Path integration provides a scaffold for landmark learning in desert ants. Curr Biol 2010; 20:1368 - 1371
- Lehrer M. Why do bees turn back and look?. J Comp Physiol A 1993; 172:549 - 563
- Zeil J, Kelber A, Voss R. Structure and function of learning flights in bees and wasps. J Exp Biol 1996; 199:245 - 252
- Zeil J. The control of optic flow during learning flights. J Comp Physiol A 1997; 180:25 - 37
- Collett TS. Making learning easy—the acquisition of visual information during the orientation flights of social wasps. J Comp Physiol A 1995; 177:737 - 747
- de Ibarra NH, Philippides A, Riabinina O, Collett TS. Preferred viewing directions of bumblebees (Bombus terrestris L.) when learning and approaching their nest site. J Exp Biol 2009; 212:3193 - 3204
- Lent DD, Graham P, Collett TS. Image-matching during ant navigation occurs through saccade-like body turns controlled by learned visual features. Proc Natl Acad Sci USA 2010; 107:16348 - 16353
- Lehrer M. Bees which turn back and look. Naturwissenschaften 1991; 78:274 - 276
- Collett TS, Rees JA. View-based navigation in Hymenoptera: Multiple strategies of landmark guidance in the approach to a feeder. J Comp Physiol A 1997; 181:47 - 58
- Zeil J, Boeddeker N, Hemmi JM. Vision and the organization of behaviour. Curr Biol 2008; 18:320 - 323