Abstract
Membrane proteins are abundant in nature and play a key role in many essential life processes. They typically span the membrane with one or more hydrophobic segments. Temporal changes in properties of such transmembrane (TM) segments often are a prerequisite for functional activity of membrane proteins. However, very little is known about the molecular nature of this important step in signaling. In a recent published work, we report the finding that both the sensing and transmission of DesK, a bacterial cold sensor, which has five TM segments, can be captured into a chimerical single membrane-spanning minimal sensor. Thus, the DesK system allows minimization of a complex phenomenon to a perfect functional system. This “minimalist” approach helped to uncover the modus operandis of a receptor for environmental cold, but also explores the use of a novel approach to study how the TM domains of a sensor protein transmit signals across membranes.
Genome sequencing data have revealed that approximately one out of four proteins encoded by DNA is a membrane protein.Citation1 These proteins play essential roles in many life processes, such as cell growth and division, uptake of food, communication between cells and sensory perception. To understand the role of membrane proteins in health and disease, knowledge of the molecular mechanism through which these proteins function is needed. This requires not only structural information about the protein itself but also information about how the lipid environment affects its structure and organization. In spite of their obvious importance, knowledge on the structural properties of membrane proteins is still relatively sparse. Even less is known about the dynamical processes that are essential for functioning. Membrane proteins come in a huge structural variety, but they have one property in common: they contain one or more hydrophobic regions with which they span the membrane, most often as a single α-helix or as a bundle of α-helices.Citation2 Many properties of membrane proteins are determined by interaction between these helices and the surrounding lipids, whereby the helices can act as sensors of the lipid environment. The mechanism by which these helices transmit signal across the membrane has long been a subject of interest. However, studying how transmembrane (TM) domains transmit signals across membranes is beset by unique challenges (reviewed in ref. Citation1) and the most interesting properties of TM helix interactions may be the least amenable to study by current techniques. For example, cells receive signals from the outside world by way of receptors that span the membrane. Although some receptors transmit the information across the membrane by means of an ion channel that allows ions into the cells, most receptors do not transmit material across the membrane. Rather, these receptors undergo conformational changes induced by the ligand or stimulus that interacts with the external part of the receptor, and these conformational changes travel across the membrane to the cytoplasmic portion of the receptor. Very little is known about the types of conformational changes used by receptors to carry out TM signaling.Citation3,Citation4
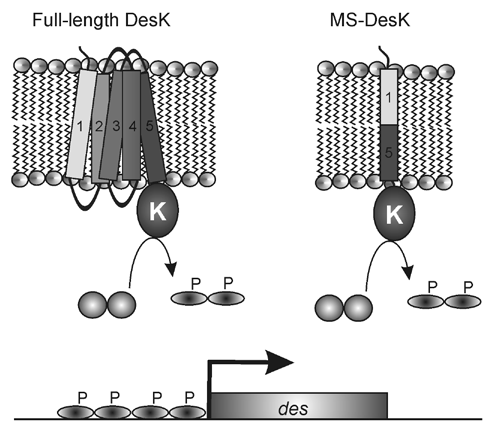
Recent discoveries on a regulation system of membrane fluidity by the molecular thermosensor DesK in Bacillus subtilis now open the way to elucidate in molecular detail how the TM segments of a signaling protein act as sensor and how they are able to transmit this information to the cytoplasmic portion of the receptor. This regulation system, which was studied by our group for over a decade, consists of a membrane-embedded cold-sensor, DesK, which reacts to temperature changes by regulating the expression of a fatty acid desaturase, Δ5Des, that inserts into the membrane and introduces unsaturated bonds in the lipids, thereby regulating membrane fluidity.Citation5–Citation8 Two recently key discoveries that helped to the elucidation of the pathway are (1) a crystallographic study of the DesK catalytic core which has revealed how the domains of this protein can interact to assemble the three active sites that determine its regulatory state, providing an excellent baseline for understanding the mechanism by which DesK function as a molecular switch that transduce bilayer deformations into protein motionsCitation9 and (2) the establishment that DesK retains its functionality even when reconstituted in pure vesicles and hence that no other protein components are involved in either sensing or signaling.Citation10 However, the mechanism that allows DesK discriminating the lipid environment to promote membrane remodeling upon a drop in ambient temperature remained elusive. In a recent report,Citation11 we showed that deletion of the first TM region (TM1) of the five-pass DesK polytopic protein abolished the ability of the resultant protein to respond to a decrease in ambient temperature and resulted in a constitutively active protein, suggesting that TM1 harbored a temperature-sensing motif. This result suggested that TM1 would detect a drop in temperature and transmit this information to TM5, which is directly connected to the catalytic core via a two-helix coiled coil, which ultimately controls the signaling state of DesK. Thus, we created a chimeric TM region consisting of the first half of TM1 and the second half of TM5, which was fused to the cytosolic domain of DesK (DesKC).
Remarkably, even after completing this exercise in molecular minimalism, the shorter TM sensor, named minimal sensor (MS), still activates effectively the kinase activity of DesKC after a temperature downshift, even when reconstituted in lipid vesicles, demonstrating that the MS domain plays a role equivalent to full-length DesK (). The MS N-terminus has an unusual motif of hydrophilic aminoacids near the lipid/water interface.Citation11 By mutating these hydrophilic residues to hydrophobic amino acids the protein was unable to stimulate the desaturase promoter, which is activated only when there is flux of phosphate from DesK to DesR upon a temperature downshift, suggesting that this motif has an important role in membrane sensing.Citation9 A likely hypothesis is that at low temperatures the membrane becomes thicker due to an increase in the lipid order, thereby trapping the hydrophilic motif inside the hydrophobic membrane environment, and thereby, due to the thermodynamic cost associated with dehydrating polar groups, somehow favoring the kinase activity of DesK. At higher temperatures the membrane lipids are more disordered and the membrane becomes thinner, allowing the hydrophilic motif to reach the aqueous environment, and resulting in phosphatase activity of DesK. If this hypothesis is correct, an increase in the length of the hydrophobic region of the TMS of MS-DesKC should increase the possibility that the hydrophilic motif becomes exposed to the aqueous phase, regardless of the growth temperature.
Indeed, introduction of 4 Val residues in the hydrophobic region of MS-DesKC was found to induce the protein to behave as a constitutive phosphatase, supporting this hypothesis.Citation9 In complementary biochemically studies, MS-DesKC was reconstituted into membrane vesicles made of phosphathidylcholines having different fatty acyl chain lengths. These experiments showed that a thicker bilayer resulted in higher autokinase activity, consistent with the hypothesis that the hydrophilic motif is a membrane thickness “ruler” device.Citation11
Several recent studies of soluble domains from receptors (and not the TMs themselves) suggest that TM motion is part of the mechanism for signal transduction. Hulko et al.Citation12 summarize four possible types of motion that have been proposed for helices in the membrane: translation, piston, rotation parallel to the membrane (pivot) and rotation perpendicular to the membrane. Such changes in the TM segments could bring about conformational changes at the cytoplasmic side of the membrane, leading to a response within the cell. Because most structural methods are compromised if there are multiple, dynamic conformations of the macromolecule of interest, it is not surprising that such motions in most membrane protein remain mysterious. Moreover, membranes are difficult to study, and their influence in signaling is still understood at the most rudimentary level.
The availability of a simplified functional signaling system, combined with recent advances in the biophysics of membranes, now allows us to elucidate the molecular nature of the changes in properties of the TM segments underlying both the sensing and the transmission mechanism by using well-defined model membranes of synthetic lipids and designed variants corresponding to TM segments of functional and non-functional minimal DesK sensors. Finally, these observations may widen our notion of what cold sensors are and how they sense temperature. With the ever-increasing number of genomes available, the search for new types of cold-sensors will benefit greatly from an improved definition of what features to look for.
Figures and Tables
Figure 1 Engineering a minimal sensor. The complete DesK sensor domain includes five TM-spanning segments that sense an increase in the order of membrane lipids promoting a kinase dominant state of DesK (K), which autophosporylates and transfer the phosphate group to DesR. Phospho-DesR activates expression of the des gene coding for a Δ5-desaturase. A truncated chimera (MS-DesK), in which the N-terminal domain of TM1 is fused to the C-terminal domain of TM5 allows near-normal control of membrane-mediated DesK signaling state.
Acknowledgements
This work was supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and Agencia Nacional de Promoción Científica y Tecnológica (FONCYT). L.E.C. and D.de M. are career investigators from CONICET. D.de M. is an international Research Scholar from the Howard Hughes Medical Institute.
Addendum to:
References
- Wallin E, von Heijne G. Genome-wide analysis of integral membrane proteins from eubacterial, archaean and eukaryotic organisms. Protein Sci 1998; 7:1029 - 1038
- Nyholm TK, Ozdirekcan S, Killian JA. How protein transmembrane segments sense the lipid environment. Biochemistry 2007; 46:1457 - 1465
- Matthews EE, Zoonens M, Engelman DM. Dynamic helix interactions in transmembrane signaling. Cell 2006; 127:447 - 450
- Cheung J, Hendrickson WA. Sensor domains of two-component regulatory systems. Curr Opin Microbiol 2010; 13:116 - 123
- Aguilar PS, Hernandez-Arriaga AM, Cybulski LE, Erazo AC, de Mendoza D. Molecular basis of thermosensing: a two-component signal transduction thermometer in Bacillus subtilis. EMBO J 2001; 20:1681 - 1691
- Cybulski LE, del Solar G, Craig PO, Espinosa M, de Mendoza D. Bacillus subtilis DesR functions as a phosphorylation-activated switch to control membrane lipid fluidity. J Biol Chem 2004; 279:39340 - 39347
- Albanesi D, Mansilla MC, de Mendoza D. The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator. J Bacteriol 2004; 186:2655 - 2663
- Mansilla MC, Cybulski LE, Albanesi D, de Mendoza D. Control of membrane lipid fluidity by molecular thermosensors. J Bacteriol 2004; 186:6681 - 6688
- Albanesi D, Martín M, Trajtenberg F, Mansilla MC, Haouz A, Alzari PM, et al. Structural plasticity and catalysis regulation of a thermosensor histidine kinase. Proc Natl Acad Sci USA 2009; 106:16185 - 16190
- Martin M, Albanesi D, Alzari PM, de Mendoza D. Functional in vitro assembly of the integral membrane bacterial thermosensor DesK. Protein Expr Purif 2009; 66:39 - 45
- Cybulski L, Martin M, Mansilla MC, Fernandez A, de Mendoza D. Membrane thickness cue for cold sensing in a bacterium. Curr Biol 2010; 20:1539 - 1544
- Hulko M, Berndt F, Gruber M, Linder JU, Truffault V, Schultz A, et al. The HAMP domain structure implies helix rotation in transmembrane signaling. Cell 2006; 126:929 - 940