Abstract
The 100 billion neurons comprising the human brain are wired together using structural extensions termed axons, dendrites, and dendritic spines. Addictive drugs remodel dendritic spine structure in certain brain regions, and with repeated exposure, induce psychomotor sensitization and impair behavioral flexibility. We recently reported that low-dose cocaine exposure, in combination with knockout of Arg—an adhesion-regulated nonreceptor tyrosine kinase that stabilizes neuronal shape starting in adolescence—recapitulates both features of chronic drug exposure in rodents. In light of these and other recent findings in the field, we suggest that cell adhesion receptors and their downstream cytoskeletal effectors act as “first responders” to psychostimulant exposure. In this model, cell adhesion factors act to stabilize existing dendritic spines in response to cocaine, and reduced expression/function is expected to increase vulnerability. Moreover, this model anticipates that increased sensitivity to psychostimulants in adolescence relates to neuronal pruning processes that occur during this developmental period.
Growth factor and adhesion receptors direct changes in cell shape and movement by acting on downstream intracellular signaling cascades that coordinate cytoskeletal dynamics. For example, integrin-mediated adhesion activates Abl family kinases to regulate cell shape and motility in several physiological contexts: fibroblast migration, breast cancer invasiveness, neuronal outgrowth and branching, and synapse and dendrite stability in the adult brain.Citation1 In primary pyramidal neurons and other cell types, Abelson-related gene (Arg) phosphorylates p190RhoGAP in a signaling cascade that ultimately results in the inhibition of the RhoA (Rho) GTPase, a master regulator of cytoskeletal rearrangement ().Citation2–Citation5 Rho activation induces neurite retraction, a process required for pruning and refining neuronal connections during development,Citation6 but inappropriate Rho activation in mature neurons leads to synapse loss and dendritic regression. Thus, integrin:Arg interactions stabilize existing synapses and the dendritic spines that house them.
Spine morphology is regulated by intrinsic biological processes, as in the case of dendritic spine pruning during post-natal development,Citation7 and external environmental stimuli such as exposure to addictive psychostimulants.Citation8,Citation9 In the ventral striatum, acute cocaine robustly increases spine density, corresponding with increased expression of PSD95, a postsynaptic marker, and the Arp3 subunit of the Arp2/3 complex, which promotes nucleation of new F-actin branches.Citation10 This early immediate spinogenic response to cocaine appears to constitute an acute burst in spines, while chronic cocaine exposure accelerates spine clearance and turnover, increasing cofilin expression, which promotes F-actin disassembly.Citation10,Citation11 Infusion of latrunculin A, a neurotoxin that results in F-actin depolymerization and spine collapse, potentiates cue-induced reinstatement, an animal model of relapse,Citation12 and blocking cocaine-induced spinogenesis using more subtle manipulations potentiates psychomotor sensitization to cocaine,Citation13,Citation14 suggesting initial cocaine-elicited striatal spine growth, as occurs early in drug exposure, protects against vulnerability to repeated drug exposure (review in ref. Citation15).
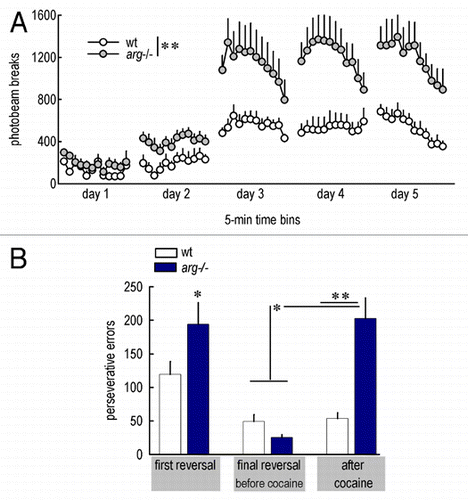
We recently reported exaggerated psychomotor and other sensitivities to cocaine in arg knockout mice, which have pre-exist- ing spine density and stability deficits.Citation5,Citation16 Our findings in these Arg-deficient mice—particularly exaggerated psychomotor sensitivity to cocaine, recapitulated in —can be interpreted as indicating that pre-existing spine deficiency results in mice that are “pre-sensitized,” and thus more vulnerable to repeated cocaine exposure and to developing reward-seeking behavior.Citation17–Citation20 An additional possibility is that cocaine sensitivity is enhanced in arg−/− mice because these animals lack a major player—Arg kinase—in the signaling cascades that would otherwise act to stabilize existing spines (via integrin-mediated adhesion) in response to cocaine. In support of this possibility, acute cocaine increases β1-integrin expression (a likely upstream Arg regulator) and decreases Rho activity (a downstream Arg effector) in the ventral striatumCitation21,Citation22—both responses would be expected to stabilize existing dendritic spines in response to cocaine and may in fact act in the same pathway, with Arg:p190RhoGAP interactions functioning as the intermediary between the two (see ). In the absence of Arg and related cytoskeletal regulatory factors, the cellular response to repeated cocaine would be biased towards spine clearance and, as we have shown, exaggerated behavioral sensitivity on multiple measures. Thus, emerging evidence, including ours, suggests spine stability may have protective properties in the face of repeated drug exposure.
The serine/threonine kinase Cdk5 has been widely regarded as a regulator of cocaine sensitivity since its original identification as a DARPP32 binding partner.Citation23 As with Arg deficiency, Cdk5 inhibition or forebrain deficiency promotes psychomotor sensitization to cocaineCitation23–Citation25 and also blunts acute cocaine-induced striatal spinogenesis.Citation26 Recent evidence indicates that, like Arg, Cdk5 acts via a p190RhoGAP-Rho-R°CK cascade, in this case mediated by Src, such that Cdk5 inhibition promotes cytoskeletal reorganization.Citation27 Thus, as we hypothesize with Arg, cocaine-mediated activation of Cdk523 may brake psychomotor sensitization by stabilizing existing spines against repeated cocaine exposure.
The majority of studies regarding mechanisms of psychostimulant-induced spine reorganization have been conducted using ventral striatal tissue extracts or microinfusion, but psychostimulant-induced spine reorganization is documented in several brain regions, including orbital subregions of the prefrontal cortex.Citation28 Human neuroimaging studies document prefrontal hypofunc- tion,Citation29–Citation32 poor impulse control and behavioral inflexibilityCitation33 in cocaine addicts. In monkeys, diminished glucose utilization in the orbital cortex is associated with both early and late phases of cocaine self-administration,Citation34,Citation35 as well as noncontingent drug administration.Citation36 These and other findings support the view that atrophy of so-called “inhibitory control” processes mediated by the prefrontal cortex and associated limbic-striatal circuits are both characteristic of addiction and promote further drug use.Citation37,Citation38 Inability of animals to mount an immediate cell adhesive/cytoskeletal response to cocaine to either maintain existing spines or generate new spines in specific regions may exacerbate this cyclical decline.
Using an instrumental “reversal learning” task in which mice must shift responding from a previously reinforced aperture to a newly reinforced aperture within an operant conditioning chamber, we tested this hypothesis and found that drug-naïve arg−/− mice showed modest deficits in reversal (recapitulated in , left), consistent with the effects of orbitofrontal lesions in mice performing the same task.Citation39 arg−/− mice could, however, acquire the reversed contingency over time, such that the number of errors was indistinguishable from wild type (wt) levels for a given reversal (, middle). High-dose psychostimulant exposure impairs performance on similar reversal tasks,Citation40–Citation42 but we administered subthreshold concentrations of cocaine to wt and arg−/− mice, such that wt mice were unaffected. arg−/− mice were, by contrast, vulnerable, executing >4-fold more errors a full week after the last cocaine exposure.Citation16 These findings, recapitulated here in at right, provide some of the first evidence that spine instability and cocaine exposure have synergistic consequences for inhibitory control processes.
As discussed above, previous studies indicate that Arg acts as part of a Rho inhibitory pathway in the brain to mitigate synapse and spine pruning processes during post-natal development, allowing for the maintenance of dendritic arbors throughout the adult life of the animal. Disruption of this pathway via the loss of Arg increases Rho activity, leading to a loss of synapses and branch points in the cortexCitation3 and hippocampusCitation5 first detectable in early adolescence. Also during early adolescence, the neurotrophin Brain-derived Neurotrophic Factor (BDNF) stimulates growth of cortical neurons by enhancing the rate of dynamic branch motility,Citation43,Citation44 and the loss of BDNF signaling through its high-affinity trkB receptor results in cortical dendrite arbor shrinkage after postnatal day 3.Citation45,Citation46 Like Arg, BDNF is thus critical for the outgrowth and maintenance of neurons in the transition from prenatal development to adulthood, and like arg−/− mice, bdnf+/− mice show heightened sensitivity to food reward as adults, which can be rescued by replacing BDNF in the orbitofrontal cortex (Gourley and Taylor, unpublished). Obviously, BDNF and Arg differ in certain ways—for example, acute activity-dependent BDNF release stimulates dendritic growth but destabilizes spines,Citation43,Citation44 presumably allowing for the growth of new spines, whereas Arg appears to primarily act as a stabilizing factor for both dendrites and spines. Nonetheless, these and other findings point to the orbital cortex in particular as a site at which disruptions in adolescent cortical development via multiple molecular targets may manifest in adulthood as hypersensitivity to reward.
These findings imply that pharmacological agents that promote cell adhesion or growth factor signaling may be effective pharmacological adjuncts to cognitive-behavioral therapies for addiction and other diseases in which cytoskeletal abnormalities are thought to play causal or contributing roles. Towards this goal, the ROCK inhibitor, fasudil, was recently shown to increase dendritic length during the prodromal period in a mouse model of Alzheimer's Disease (see again ).Citation47 This compound is clinically approved in Japan to treat cerebral vaso- spasm,Citation48 suggesting it could be safely adopted as a treatment adjunct in addiction. One caveat, however, is that manipulation of putative targets may be expected to have site- or cell type-selective effects that could complicate the development of novel pharmacotherapies. For example, while orbitofrontal BDNF deficiency appears to confer hyper-sensitivity to appetitive reward in the context of instrumental responding for food (as described above) or in terms of passive consumption of palatable foods,Citation49 selective gene knockdown in dorsomedial prefrontal subregions has the opposite effects.Citation50 These subregions supply the striatum with BDNF via anterograde transport,Citation51 and recent studies indicate that BDNF plays unique and specific roles in the postnatal growth, development and maturation of striatal neurons.Citation52 Moreover, BDNF-mediated cocaine sensitivity appears to be differentially affected by the activation of dopamine D1- relative to D2-containing cells, which are largely segregated in striatal systems.Citation53 This profile may account for evidence that BDNF expression and signaling within the striatum promotes—rather than brakes, as in the case of Arg—psychomotor sensitization to cocaine.Citation54
A role for cell adhesion and growth factor signaling in acute reactivity, and subsequent vulnerability, to cocaine is still being established, but what our previous report,Citation16 as well as others,Citation12–Citation14 suggest is that disturbances in the processes that act to stabilize synapses, spines and dendritic arbor engender vulnerability to both the rewarding and deleterious properties of repeated psychostimulant exposure. That arg−/− mice also lack sensitivity to haloperidol on orbitofrontal-dependent behavioral tasks raises the possibility that adolescent-onset vulnerabilities relate to frontal dopamine D2 receptor expression patterns.Citation16 These findings also have implications for efficacy of antipsychotic drugs acting on the dopamine system, as neuroplasticity within the orbital cortex may be associated with therapeutic-like outcomes.Citation55
The median age of first illicit drug use among psychostimulant addicts is 16 years, with few adult addicts having first administered their drug of choice after the age of 20.Citation56 Given that cortical development culminates during these adolescent and peri-adolescent periods, further characterization of the molecular mechanisms that regulate cortical neuronal shape and complexity and their impact upon sensitivity to drugs of abuse may provide a significant advance towards a more comprehensive view of the cyclical behavioral patterns that characterize addiction, as well as insight into early intervention techniques and pharmacotherapies.
Abbreviations
| Abl | = | abelson |
| Arg | = | abelson-related gene |
| Arp2/3 | = | actin-related proteins 2/3 |
| BDNF | = | brain-derived neurotrophic factor |
| Cdk5 | = | cyclin-dependent kinase 5 |
| D1 | = | dopamine receptor type 1 |
| D2 | = | dopamine receptor type 2 |
| DARPP32 | = | dopamine and cAMP-regulated phosphoprotein 32 |
| F-actin | = | filamentous actin |
| PSD95 | = | post-synaptic density 95 |
| ROCK | = | Rho kinase |
| Rho | = | RhoA GTPase |
| trkB | = | tyrosine receptor kinase B |
Figures and Tables
Figure 1 Arg interacts with β-integrin tails and p190RhoGAP to stabilize synapses, spines and dendrites. Arg is activated through a physical interaction with intracellular β-integrin tails, which allows for p190RhoGAP phosphorylation and recruitment to the membrane by p120RasGAP. This complex inhibits RhoA GTPase activity. In the absence of RhoA inhibition, RhoA acts on ROCK to destabilize the actin cytoskeleton, leading to spine and dendrite collapse and synapse loss. Conversely, ROCK inhibition elongates dendritic branches.Citation47
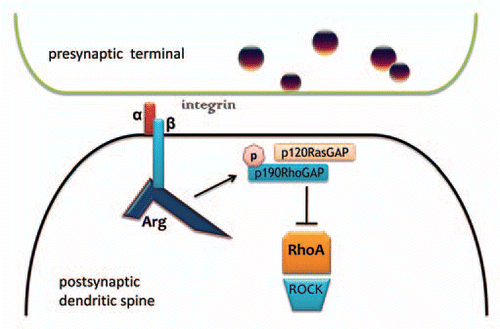
Figure 2 Arg deficiency confers vulnerability to cocaine and cocaine-induced deficits in a reversal task. (A) Arg-deficient mice show heightened psychomotor sensitivity to cocaine, as indicated by greater photobeams broken in a locomotor monitoring apparatus after low-dose (10 mg/kg, i.p.) cocaine administered across several days. Locomotor activity in the absence of cocaine is unchanged (not shown; Citation5,Citation16). Because repeated breaking of the same photobeam constituted the largest difference between wt and arg−/− mice, these specific counts (suggestive of stereotypy) are shown here. (B) During a reversal test in which mice must redirect responding from a previously reinforced operant aperture to a previously non-reinforced aperture, arg−/− mice initially generate more “perseverative” errors than wild type (wt) counterparts (left), consistent with cortical dendritic simplification in these mice. arg−/− mice acquired the reversal with repeated training (middle), but repeated exposure to low-dose cocaine greatly exacerbated perseverative errors (right), despite a prolonged washout period prior to test. *p ≤ 0.05; **p < 0.001.
Acknowledgements
Work in our laboratories is supported by PHS grants NS039475, CA133346 (A.J.K.) and DA011717 (J.R.T.); the Connecticut Department of Mental Health and Addiction Services (J.R.T.); and the Interdisciplinary Research Consortium on Stress, Self-control and Addiction (UL1-DE19586 and the NIH Roadmap for Medial Research/Common Fund, AA017537) (S.L.G., J.R.T., A.J.K.). AJK is also an Established Investigator of the American Heart Association.
References
- Bradley WD, Koleske AJ. Regulation of cell migration and morphogenesis by Abl-family kinases: emerging mechanisms and physiological contexts. J Cell Sci 2009; 122:3441 - 3454
- Hernández SE, Settleman J, Koleske AJ. Adhesion-dependent regulation of p190RhoGAP in the developing brain by the Abl-related gene tyrosine kinase. Curr Biol 2004; 14:691 - 696
- Moresco EMY, Donaldson S, Williamson A, Kolese AJ. Integrin-mediated dendrite branch maintenance requires abelson (Abl) family kinases. J Neurosci 2005; 25:6105 - 6118
- Bradley WD, Hernαndez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120Ras-GAP and recruitment to the membrane. Mol Biol Cell 2006; 17:4827 - 4836
- Sfakianos MK, Eisman A, Gourley SL, Bradley WD, Scheetz AJ, Settleman J, et al. Inhibition of Rho via Arg and p190RhoGAP in the postnatal mouse hippocampus regulates dendritic spine maturation, synapse and dendrite stability and behavior. J Neurosci 2007; 27:10982 - 10992
- Ruchhoeft ML, Ohnuma S, McNeill L, Holt CE, Harris WA. The neuronal architecture of Xenopus retinal ganglion cells is sculpted by rho-family GTPases in vivo. J Neurosci 1999; 19:8454 - 8463
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 2000; 24:417 - 463
- Robinson TE, Kolb B. Persistent structural modifications in nucleus accumbens and prefrontal cortex neurons produced by previous experience with amphetamine. J Neurosci 1997; 17:8491 - 8497
- Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology 2004; 47:33 - 46
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci 2009; 29:2876 - 2884
- Pollard TD. Regulation of actin filament assembly by Arp2/3 complex and formins. Annu Rev Biophys Biomol Struct 2007; 36:451 - 477
- Toda S, Shen HW, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci 2006; 26:1579 - 1587
- Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron 2008; 59:621 - 633
- Kiraly DD, Ma XM, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry 2010; 68:249 - 255
- Chandler LJ, Kalivas PW. Brain's defence against cocaine. Nature 2008; 455:743 - 744
- Gourley SL, Koleske AJ, Taylor JR. Loss of dendrite stabilization by the Abl-related gene (Arg) kinase regulates behavioral flexibility and sensitivity to cocaine. Proc Natl Acad Sci USA 2009; 106:16859 - 16864
- Taylor JR, Robbins TW. Enhanced behavioural control by conditioned reinforcers following microinjections of d-amphetamine into the nucleus accumbens. Psychopharmacology 1984; 84:405 - 412
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology (Berl) 1999; 142:31 - 40
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”). Biol Psychiatry 2001; 50:137 - 143
- Robinson TE, Berridge KC. The psychology and neurobiology of addiction: an incentive-sensitization view. Addiction 2000; 95:91 - 117
- Wiggins AT, Pacchioni AM, Kalivas PW. Integrin expression is altered after acute and chronic cocaine. Neurosci Lett 2009; 450:321 - 323
- Kim WY, Shin SR, Kim S, Jeon S, Kim JH. Cocaine regulates ezrin-radixin-moesin proteins and RhoA signaling in the nucleus accumbens. Neuroscience 2009; 163:501 - 505
- Bibb JA, Chen J, Taylor JR, Svenningsson P, Nishi A, Snyder GL, et al. Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 2001; 410:376 - 380
- Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. Proc Natl Acad Sci USA 2007; 104:4147 - 4152
- Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, et al. Cdk5 modulates cocaine reward, motivation and striatal neuron excitability. J Neurosci 2007; 27:12967 - 12976
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience 2003; 116:19 - 22
- Tripathi BK, Zelenka PS. Cdk5-dependent regulation of Rho activity, cytoskeletal contraction and epithelial cell migration via suppression of Src and p190RhoGAP. Mol Cell Biol 2009; 29:6488 - 6499
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson PV. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cereb Cortex 2004; 15:341 - 348
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, et al. Long-term frontal brain metabolic changes in cocaine abusers. Synapse 1992; 11:184 - 190
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, et al. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 1993; 14:169 - 177
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 2000; 10:318 - 325
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, et al. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 2001; 158:2015 - 2021
- Rogers RD, Robbins TW. Investigating the neurocognitive deficits associated with chronic drug misuse. Curr Opin Neurobiol 2001; 11:250 - 257
- Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, et al. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci 2002; 22:7687 - 7694
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci 2006; 23:3109 - 3118
- Lyons D, Friedman DP, Nader MA, Porrino LJ. Cocaine alters cerebral metabolism within the ventral striatum and limbic cortex of monkeys. J Neurosci 1996; 16:1230 - 1238
- Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology 1999; 146:373 - 390
- Robbins TW, Everitt BJ. Drug addiction: bad habits add up. Nature 1999; 398:567 - 570
- Gourley SL, Lee AS, Howell JL, Pittenger C, Taylor JR. Dissociable regulation of goal-directed action within mouse prefrontal cortex. Eur J Neurosci 2010; 32:1726 - 1734
- Jentsch JD, Olausson P, De La Garza R 2nd, Taylor JR. Impairments of reversal learning and response perseveration after repeated, intermittent cocaine administrations to monkeys. Neuropsychopharmacology 2002; 26:183 - 190
- Schoenbaum G, Saddoris MP, Ramus SJ, Shaham Y, Setlow B. Cocaine-experienced rats exhibit learning deficits in a task sensitive to orbitofrontal cortex lesions. Eur J Neurosci 2004; 19:1997 - 2002
- Calu DJ, Stalnaker TA, Franz TM, Singh T, Shaham Y, Schoenbaum G. Withdrawal from cocaine self-administration produces long-lasting deficits in orbitofrontal-dependent reversal learning in rats. Learn Mem 2007; 14:325 - 328
- Horch HW, Krüttgen A, Portbury ST, Katz LC. Destabilization of cortical dendrites and spines by BDNF. Neuron 1999; 23:353 - 364
- Horch HW, Katz LC. BDNF released from single cells elicits local dendritic growth in nearby neurons. Nat Neurosci 2002; 5:1177 - 1184
- Xu B, Zang K, Ruff NL, Zhang YA, McConnell SK, Stryker MP, et al. Cortical degeneration in the absence of neurotrophin signaling: Dendritic retraction and neuronal loss after removal of the receptor trkB. Neuron 2000; 26:233 - 245
- Gorski JA, Zeiler SR, Tamowski S, Jones KR. Brainderived neurotrophic factor is required for the maintenance of cortical dendrites. J Neurosci 2003; 23:6856 - 6865
- Couch BA, DeMarco GJ, Gourley SL, Koleske AJ. Increased dendrite branching in AβPP/PS1 mice and elongation of dendrite arbors by fasudil administration. J Alzheimers Dis 2010; 20:1003 - 1008
- Shibuya M, Suzuki Y, Sugita K, Saito I, Sasaki T, Takakura K, et al. Effect of AT877 on cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Results of a prospective placebo-controlled double-blind trial. J Neurosurg 1992; 76:571 - 577
- Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J 2000; 19:1290 - 1300
- Gourley SL, Howell JL, Rios M, DiLeone RJ, Taylor JR. Prelimbic cortex bdnf knock-down reduces instrumental responding in extinction. Learn Mem 2009; 16:756 - 760
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, et al. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature 1997; 389:856 - 860
- Rauskolb S, Zagrebelsky M, Dreznjak A, Deogracias R, Matsumoto T, Wiese S, et al. Global deprivation of brain-derived neurotrophic factor in the CNS reveals an area-specific requirement for dendritic growth. J Neurosci 2010; 30:1739 - 1749
- Lobo MK, Covington HE, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 2010; 330:385 - 390
- Horger BA, Iyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci 1999; 19:4110 - 4122
- Homayoun H, Moghaddam B. Orbitofrontal cortex neurons as a common target for classic and glutamatergic antipsychotic drugs. Proc Natl Acad Sci USA 2008; 105:18041 - 18046
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry 2003; 160:1041 - 1052