Abstract
Cave animals are faced with the challenge of carrying out fundamental life processes in a completely dark environment. Evolution of behavioral changes could be one of the key steps that adapt these animals to the absence of light. Astyanax mexicanus is a teleost with sighted surface dwelling (surface fish) and blind cave dwelling (cavefish) forms. Cavefish, a descendant of surface fish ancestors, have evolved a suite of constructive traits including an increase in the number and size of superficial neuromasts (SN). Prior to our study, no clear relationships had been established between constructive traits and the evolution of behavior. The current results link SN enhancement to vibration attraction behavior (VAB), a behavioral shift that is beneficial for feeding in a dark environment. We discuss a possible scenario in which the evolution of VAB may be a key factor in the establishment and survival of cavefish ancestors in the dark cave environment.
Cave animals have evolved a suite of characteristic morphological and physiological changes, including the regression of eyes and pigmentation, lengthening of appendages, enhanced mechanosensory, gustatory and olfactory sensory systems, low metabolic rates and high longevity, which are collectively known as troglomorphism.Citation1 These animals have also evolved behavioral changes to navigate their unusual habitats, detect animate and inanimate food items, and distinguish mates in the absence of vision.Citation2–Citation5 The relationship between these behavioral changes and the constructive evolution of troglomorphic characters, particularly non-visual sensory systems, is poorly understood. Most studies of cave animals have focused on the disappearance of traits (eyes and pigmentation) rather than on the biology of constructive beneficial traits.Citation6–Citation11
Astyanax mexicanus is an emerging model organism for understanding the evolution of behavior and sensory systems during adaptation to a novel environment.Citation12,Citation13 Within the past few million years, at least 29 geographically isolated Astyanax cavefish populations were established by two or three radiations of ancestral surface fish and their subsequent isolation in limestone caves in the Sierra de El Abra region of northeastern Mexico.Citation6,Citation9,Citation14 Some of these cavefish populations have evolved troglomorphic traits independently.Citation15,Citation16
In our study, we have focused on vibration attraction behavior (VAB), the attraction of cavefish to a source of water vibration in a cave pool. Several lines of evidence support the conclusion that the neuromast sensory system, and SN in particular, are involved in VAB. First, sensitivity to cobalt and gentamicin implicate neuromasts rather than the inner ear in this behavior. Second, the vibration frequency for evoking maximal VAB is 35 Hz, which is within the best sensing range of Astyanax SN, but does not coincide with the maximal vibration frequency recognized by canal neuromasts (CN) or the inner ear.Citation17,Citation18 Third, the ontogeny of VAB matches the timing of SN development. Fourth, ablation of SN in the cavefish head or trunk region significantly reduces VAB, demonstrating a critical role of SN in this behavior. Finally, F1 hybrids generated by a surface fish × cavefish cross show intermediate numbers of SN and levels of VAB, substantiating the conclusion that high levels of VAB are caused by SN enhancements.
Evolution of an Adaptive Behavior and its Ecological Relevance
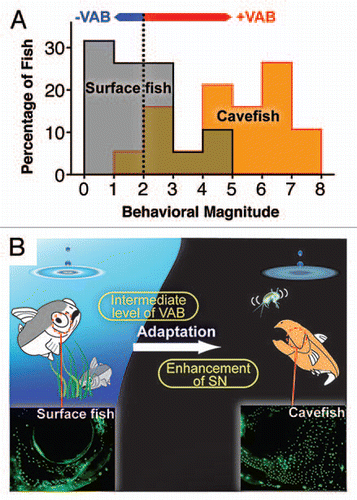
The response to water vibrations mediated by VAB may be one of the beneficial traits cavefish have evolved to feed more effectively in the cave environment. We have capitalized on the existence of a relatively small number of surface fish with VAB and cavefish lacking VAB () to demonstrate the advantage of this behavioral shift for prey capture. The results showed that surface fish or cavefish with VAB predominate in prey capturing activity over those lacking VAB in the dark, but not in the light, suggesting that this behavior has adaptive significance in caves.
In the cave environment, which lacks large predators, cavefish may be free to express behaviors that would be risky or catastrophic in lighted habitats. Astyanax surface fish have a known nocturnal predator, the prawn Macrobrachium,Citation19 supporting the possibility that surface fish exhibiting VAB could be exposed to predation in the wild. Nevertheless, a small proportion of laboratory raised surface fish have been detected with an intermediate level of VAB, which could be abolished by lateral line inhibitors, suggesting that the VAB phenotype is present at low frequencies in natural populations. Once introduced into a dark cave, surface fish with VAB would have an advantage over those lacking this phenotype, and therefore these individuals could serve as the founders of cavefish populations (). The enhancement of SN and VAB tuning to 35 Hz might occur in a second evolutionary step after surface fish have already entered caves and natural selection is operating, considering the fact that surface fish individuals with VAB do not show a large increase of SN or tuning at 35 Hz (surface fish VAB ranges from 5–35 Hz without a peak). After the second stage of VAB evolution, cavefish with enhanced SN and VAB tuned to 35 Hz would be able to detect prey more efficiently in the dark ( and see below). This scenario is perhaps one of the ways in which Astyanax became adapted to caves and eventually evolved into cavefish. Our results underscore the importance of behavioral diversity in adapting animals to new environmental challenges.
Cavefish probably feed on a variety of stationary and moving items in cave pools. Stationary objects located at the bottom of cave pools, such as particles of bat guano, could be efficiency detected and consumed using olfactory cues and the specialized feeding posture behavior that has evolved in cavefish.Citation12,Citation20,Citation21 In contrast, VAB may direct cavefish to moving prey and other disturbances in the water column. Small invertebrates, such as copepods, can produce 30–40 Hz vibrations,Citation22 and may be present in water dropping from the cave ceiling.Citation23 It has been estimated that a water droplet falling from a height of 1 to 10 m produces a 40 to 60 Hz frequency,Citation24 which is near the peak of cavefish VAB. Interestingly, Astyanax cavefish in motion produce a 30–90 Hz turbulence,Citation25 suggesting that cavefish feeding activities could also produce water disturbances that other cavefish could follow.Citation26 Therefore, cavefish feeding activity could evoke VAB and alert other cavefish to the presence of food.
Tuning to 35 Hz can be explained by the length and stiffness of the SN cupula, which could change the range of sensing ability for flow speed.Citation27 Cavefish have much longer cupulae (300 µm) than surface fish (40 µm),Citation28 which could help cavefish detect lower frequency water fluctuations by penetrating the hydrodynamic boundary layer formed on the fish surface. Alternatively, evolutionary changes in lateral line processing centers in the central nervous system may mediate tuning. In future studies, combining neurobiological and genetic approaches may reveal the physiological mechanisms related to the evolution of VAB.
Figures and Tables
Figure 1 A proposed scenario for adaptation to life in caves mediated by VAB. (A) VAB levels in surface fish and cavefish indicated by behavioral magnitude. Behavioral magnitude is the square root of the number of approaches to a vibrating rod (35 Hz) during a 3 min assay period. Surface fish: n = 19, gray area; cavefish: n = 19, orange area. Green area: overlap between surface and cavefish. Vertical dashed line represents the cutoff value for classifying fish with (above 2) or without (below 2) VAB. The red horizontal bar indicates the range of surface fish individuals with an intermediate level of VAB, which dominate over surface fish without VAB in competitive prey capture assays. The blue horizontal bar indicates the range of cavefish individuals without VAB, which are out-competed by cavefish with VAB in prey capture assays. (B) A diagrammatic summary of the enhancement of VAB and SN during adaptation of Astyanax to life in caves. Ovals on the top left and right indicate ripples produced by a dropping object (percolating water), which may contain vibrating prey (indicated in right side above cavefish). Lower parts show neuromasts stained by the fluorescent DASPE I in the cranial regions of surface fish (left) and cavefish (right). Surface fish with intermediate levels of VAB dominate in prey capturing but do not show a large increase of SN. Thus, the first adaptive step is proposed to occur by expressing an intermediate level of VAB, and SN elaboration is suggested to enhance and fine-tune VAB during the second step of adaptation to cave life.
Acknowledgements
A Japan Society for the Promotion of Science Postdoctoral Fellowship to M.Y. and NIH (R01-EYE014619) and NSF (IBN-052384) grants to W.R.J. supported this research.
Addendum to:
References
- Culver DC. Cave life, Evolution and ecology 1982; Cambridge Harvard University Press
- Sharma S, Coombs S, Patton P, de Perera TB. The function of wall-following behaviors in the Mexican blind cavefish and a sighted relative, the Mexican tetra (Astyanax). J Comp Physiol A, Neuroethol Sens Neural Behav Physiol 2009; 195:225 - 240
- Plath M, Rohde M, Schröder T, Taebel-Hellwig A, Schlupp I. Female mating preferences in blind cave tetras Astyanax fasciatus (Characidae, Teleostei). Behaviour 2006; 143:15 - 32
- de Perera TB. Fish can encode order in their spatial map. Proc R Soc Lond B, Biol Sci 2004; 271:2131 - 2134
- Coombs S, Görner P, Münz H. The mechanosensory lateral line 1989; New York Springer-Verlag
- Borowsky R. Restoring sight in blind cavefish. Curr Biol 2008; 18:23 - 24
- Jeffery WR. Evolution and development in the cavefish Astyanax. Curr Top Dev Biol 2009; 86:191 - 221
- Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, et al. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat Genet 2006; 38:107 - 111
- Wilkens H. Regressive evolution: ontogeny and genetics of cavefish eye rudimentation. Biol J Linn Soc 2007; 92:287 - 296
- Yamamoto Y, Byerly MS, Jackman WR, Jeffery WR. Pleiotropic functions of embryonic sonic hedgehog expression link jaw and taste bud amplification with eye loss during cavefish evolution. Dev Biol 2009; 330:200 - 211
- Gross JB, Borowsky R, Tabin CJ. A novel role for Mc1r in the parallel evolution of depigmentation in independent populations of the cavefish Astyanax mexicanus. PLoS Genet 2009; 5:1000326
- Wilkens H. Evolution and genetics of epigean and cave Astyanax-fasciatus (Characidae, Pisces)—Support for the neutral mutation theory. Evol Biol 1988; 23:271 - 367
- Jeffery WR. Emerging model systems in evo-devo: cavefish and microevolution of development. Evol Dev 2008; 10:265 - 272
- Ornelas-García CP, Domínguez-Domínguez O, Doadrio I. Evolutionary history of the fish genus Astyanax Baird & Girard (1854) (Actinopterygii, Characidae) in Mesoamerica reveals multiple morphological homoplasies. BMC Evol Biol 2008; 8:340
- Dowling TE, Martasian DP, Jeffery WR. Evidence for multiple genetic forms with similar eyeless phenotypes in the blind cavefish, Astyanax mexicanus. Mol Biol Evol 2002; 19:446 - 455
- Strecker U, Bernatchez L, Wilkens H. Genetic divergence between cave and surface populations of Astyanax in Mexico (Characidae, Teleostei). Mol Ecol 2003; 12:699 - 710
- Münz H. Coombs S, Görner P, Münz H. Functional organization of the lateral line periphery. The mechanosensory lateral line 1989; New York Springer-Verlag
- Popper AN. Auditory capacities of the Mexican blind cave fish (Astyanax jordani) and its eyed ancestor (Astyanax mexicanus). Anim Behav 1970; 18:552 - 562
- Wilson EV, Thomas RQ, Evans LM. Macrobrachium as a possible determinant of Astyanax fasciatus distribution in a neotropical lowland stream. Dartmouth Stud Trop Ecol 2004; 2004:99 - 102
- Protas M, Tabansky I, Conrad M, Gross JB, Vidal O, Tabin CJ, et al. Multi-trait evolution in a cave fish, Astyanax mexicanus. Evol Dev 2008; 10:196 - 209
- Schemmel C. Studies on the genetics of feeding behavior in the cave fish Astyanax mexicanus f. anoptichthys. an example of apparent monofactorial inheritance by polygenes. Z Tierpsychol 1980; 53:9 - 22
- Montgomery JC, Macdonald JA. Sensory tuning of lateral line receptors in antarctic fish to the movements of planktonic prey. Science 1987; 235:195 - 196
- Culver DC, Pipan T. The biology of caves and other subterranean habitats 2009; Oxford Oxford University Press
- Pumphrey HC, Walton AJ. Experimental study of the sound emitted by water drops impacting on a water surface. Eur J Phys 1988; 9:225 - 231
- Bleckmann H, Breithaupt T, Blickhan R, Tautz J. The time course and frequency content of hydrodynamic events caused by moving fish, frogs and crustaceans. J Comp Physiol A 1991; 168:749 - 757
- Hüppop K. Food-finding ability in cave fish. Int J Speleol 1987; 16:59 - 66
- McHenry MJ, Strother JA, van Netten SM. Mechanical filtering by the boundary layer and fluid-structure interaction in the superficial neuromast of the fish lateral line system. J Comp Physiol A, Neuroethol Sens Neural Behav Physiol 2008; 194:795 - 810
- Teyke T. Morphological differences in neuromasts of the blind cave fish Astyanax hubbsi and the sighted river fish Astyanax mexicanus. Brain Behav Evol 1990; 35:23 - 30