Abstract
The epsin family of endocytic adaptors has been found to be upregulated in cancer; however the relevance of these findings to this pathological condition is unclear. We have recently demonstrated that epsins are required for cell migration. In fact, epsin overexpression promotes cancer cell invasion. Further, and in agreement with our previous findings, we also observed that overexpression of epsins led to epithelial cell migration beyond colony boundaries. Additionally, our results show that epsin-3 is the most potent paralog enhancing cell migration and invasion. Interestingly, epsin-3 expression is not widespread but highly restricted to migratory keratinocytes and aggressive carcinomas. Upon further investigation, we also identified epsin-3 as being expressed in pancreatic cancer cells. These findings suggest that upregulation of the EPN3 gene is specifically associated with invasive, aggressive cancers. We predict that investigation of these links between the endocytic machinery and mechanisms involved in tumor dissemination will contribute to the development of novel anti-metastatic and anti-cancer strategies.
The Epsin Family of Endocytic Adaptors Promote Cancer Cell Invasion
It is widely accepted that functional abnormalities in the endocytic machinery can lead to the onset of malignant transformation. In its most straightforward interpretation, lack of function of endocytic proteins would lead to deficient endocytosis and therefore to prolonged signaling from activated receptors. Interestingly, downregulation of the expression levels of endocytic proteins such as Dab2, Numb and POB1 have been observed in several cancers including ovarian, prostate and breast cancer.Citation1–Citation5 Another mechanism by which abnormal endocytic protein function can lead to carcinogenesis is through the generation of aberrant fusion pro- teins.Citation6 For example, chromosomal translocations involving the CALM (Clathrin Assembly Lymphoid Myeloid leukemia) and AF10 (ALL1 Fused 10) genes produce a fusion protein implicated in acute leukemia.Citation7
Nevertheless, there are several examples of endocytic proteins upregulated in cancer. For example, elevated levels of epsins have been reported to be augmented in skin, breast and lung cancer.Citation8–Citation10 Additionally, intersectin has been shown to induce fibroblast transformation in vitro.Citation11 Interestingly, both endocytic proteins have been directly implicated in the activation of Rho GTPase signaling pathways. Specifically, whereas the intersectin-L isoform has intrinsic Cdc42 GEF activity, epsins bind and inhibit the function of GAPs for Cdc42 and Rac1.Citation12 Although it is not completely clear if amplified RhoGTPase signaling is sufficient to induce malignant transformation, it is predicted to enhance the dissemination of cancer. Indeed, we have demonstrated that the epsin family of endocytic adaptors is required for cell migrationCitation13 and that this function depends on the interaction of these proteins with the Cdc42/Rac1 GAP and Ral effector RalBP1.Citation13 Further, our studies indicate that epsin-RalBP1 complex formation is required for proper Rac1 signaling.Citation13
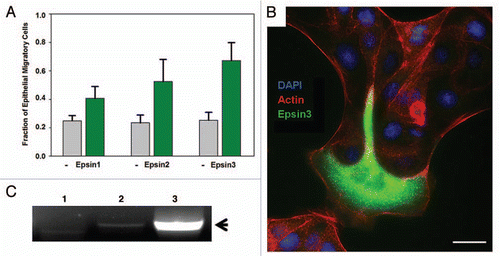
RalBP1 has been observed to be highly upregulated in several invasive cancers including bladder, lung, prostate and skin cancerCitation14,Citation15 and implicated in cancer cell migration, spreading and survival.Citation16,Citation17 It should be noted that epsins, particularly epsin-3, are upregulated in breast and skin cancer.Citation9,Citation10 Importantly, either epsin or RalBP1 overexpression lead to enhanced cell invasion through the basement membrane.Citation13 This observation suggests that enhanced expression of these endocytic proteins contribute to cancer aggressiveness by promoting cell invasion. In agreement with this prediction, we have observed morphological changes in MDCK epithelial cells upon overexpression of epsin-2 and epsin-3 which indicate enhanced cell migration (). Specifically, epsin-transfected cells repeatedly extend lamellipodia beyond the colony boundaries in a way which closely resembles epithelial leader cell migrationCitation18 but they also can be found migrating out of epithelial colonies entirely (). Interestingly, a strikingly similar transition in MDCK behavior has been observed previously upon overexpression of the Arf6 GEF ARNO.Citation19 ARNO overexpression causes the extension of broad lammelipodia and enhanced cell migration which can be attributed to enhanced activation of both Arf6 and Rac1.Citation19 Our previous findings show that the epsin family of adaptors is also signaling to promote Arf6 and Rac1 activation, suggesting that these independent results are obtained by the activation of similar GTPase signaling pathways.Citation13
We consistently observed that epsin-3 was the most potent paralog for inducing enhancement of cell invasionCitation13 and MDCK migration (). Interestingly, epsin-3 has a limited expression pattern, essentially restricted to migratory cells and basal carcinomas.Citation9 In fact, epsin-3 expression is highly upregulated in breast cancer cell lines.Citation9,Citation10 Further, we have also identified epsin-3 as being expressed in mouse pancreatic cancer modelsCitation13 and in invasive human pancreatic cell lines such as BxPC-3 (). These findings suggest that upregulation of the EPN3 gene is specifically associated with invasive cancer. Our laboratory is currently engaged in further investigations required to prove or disprove this hypothesis.
Perspectives
Additional epsin-dependent mechanisms for the enhancement of cancer cell invasion.
Although our data indicate that the ability of epsin to affect cell invasion is mediated by its interaction with RalBP1 and the resulting RhoGTPase activation,Citation13 we cannot discard additional contributions by other mechanisms. Indeed, endocytosis itself has been proposed to play an important role during cell migration. Thus, defects in the function of endocytic proteins such as Dab2, ARH, Numb, AP2 and clathrin, have also been linked to abnormal cell migration due to defective integrin endocytosis.Citation20–Citation23
Additionally, epsin has been directly and specifically connected to the activation of the Notch signaling pathwayCitation24,Citation25 which is known to be involved in cell migration/invasion.Citation26 In Drosophila, epsin is the only endocytic adaptor necessary for activation of Notch signaling in signal sending cells, likely due to its special ability to internalize ubiquitinated Notch-ligands.Citation24,Citation25 Further, this Notch-signaling activation function has been shown to be conserved in worms and mice.Citation27,Citation28 Nevertheless, this juxtacrine cell-to-cell mechanism is unlikely to be involved in the epsinmediated enhancement of fibrosarcoma cell migration and invasion.Citation13 The epsin-3′s prevalent effects over other paralogs' cannot be explained by this mechanism. Specifically, since Notch-ligand internalization is a ubiquitin-dependent process,Citation29 all epsin paralogs (which bear functional ubiquitin-interacting motifs) are predicted to be equally effective in promoting cell invasion enhancement. However, it is possible that an epsin-induced, Notch-dependent mechanism operates in the context of multi-cellular environments, such as pancreatic acini.
Nevertheless, the contributions of epsin-mediated enhancement of cancer cell invasion due to endocytosis in general, and of Notch-ligands in particular, still needs to be assessed.
Cell sensitivity to anti-cancer drugs.
Metastatic cells are usually associated with enhanced resistance to chemotherapy.Citation30 Therefore, factors or pathways that contribute to migratory behavior are of high interest for therapeutic purposes. Given our recent findings, epsins rightfully join the list of potential targets for anti-metastatic and anti-cancer strategies, which already includes their interaction partner RalBP1. In fact, it is tempting to speculate that in addition to other proposed mechanisms,Citation17 RalBP1's ability to promote cancer cell survival is related to its capability of inducing migratory behavior. Therefore, function impairment of endocytic proteins crucial to cell invasion (such as epsins and RalBP1) represents an exciting new direction for developing effective cancer therapeutics.
Figures and Tables
Figure 1 Epsin overexpression induces migratory behavior in epithelial cells. MDCK cells were transiently transfected with GFP-Epsin1, 2 and 3. After transfection, cells were trypsinized and seeded on glass coverslips for 24 hr at low density to promote the formation of colonies containing approximately 50 cells. The coverslips were then fixed, co-stained with rhodamine-phalloidin and DAPI, and imaged by epifluorescence microscopy. (A) Fraction of cells at the colony periphery acquiring migratory behavior was quantified in three independent experiments. Results for epsin-transfectants and untransfected (−) cells are indicated as the Mean ± SEM . (B) Example of epithelial cells transfected with GFP-epsin-3 displaying migratory behavior. Scale bar: 20 microns. (C) RNA prepared from HeLa (1), Panc-1 (2) and BxPC-3 (3) cell lines was used as template for RT-PCR with human epsin-3 specific primers. Arrow points to epsin-3 cDNA specific fragment.
Acknowledgements
We thank Debarati Mukherjee (Purdue University) for critical reading of the manuscript and various colleagues for insightful discussions and suggestions. This project was supported by start-up funds from the Dept. of Biological Sciences, Purdue University, an American Cancer Society Institutional Research Grant through the Purdue Center for Cancer Research and a SIRG Award to R.C.A.
Addendum to:
References
- Xu XX, Yi TL, Tang B, Lambeth JD. Disabled-2 (Dab2) is an SH3 domain-binding partner of Grb2. Oncogene 1998; 16:1561 - 1569
- Schwahn DJ, Medina D. p96, a MAPK-related protein, is consistently downregulated during mouse mammary carcinogenesis. Oncogene 1998; 17:1173 - 1178
- Tseng CP, Ely BD, Li YM, Pong RC, Hsieh JT. Regulation of rat DOC-2 gene during castration-induced rat ventral prostate degeneration and its growth inhibitory function in human prostatic carcinoma cells. Endocrinology 1998; 139:3542 - 3553
- Oosterhoff JK, Penninkhof F, Brinkmann AO, Grootegoed JA, Blok LJ. REPS2/POB1 is downregulated during human prostate cancer progression and inhibits growth factor signaling in prostate cancer cells. Oncogene 2003; 22:2920 - 2925
- Colaluca IN, Tosoni D, Nuciforo P, Senic-Matuglia F, Galimberti V, Viale G, et al. NUMB controls p53 tumour suppressor activity. Nature 2008; 451:76
- Crosetto N, Tikkanen R, Dikic I. Oncogenic breakdowns in endocytic adaptor proteins. FEBS Letts 2005; 579:3231 - 3238
- Huh JY, Chung S, Oh D, Kang MS, Eom HS, Cho EH, et al. Clathrin assembly lymphoid myeloid leukemia-AF10-positive acute leukemias: a report of 2 cases with a review of the literature. Kor J Lab Med 2010; 30:117 - 121
- Spradling KD, McDaniel AE, Lohi J, Pilcher BK. Epsin 3 is a novel extracellular matrix-induced transcript specific to wounded epithelia. J Biol Chem 2001; 276:29257 - 29267
- Wang YH, Dai ZY, Sadee WG, Hancock WS. A pharmacoproteomics study of the cancer cell line EKVX using capillary-LC/MS/MS. Mol Pharmaceut 2006; 3:566 - 578
- Pawlowski KM, Krol M, Majewska A, Badowska-Kozakiewicz A, Mol JA, Malicka E, et al. Comparison of cellular and tissue transcriptional profiles in canine mammary tumor. J Physiol Pharmacol 2009; 60:85 - 94
- Wang JB, Wu WJ, Cerione RA. Cdc42 and Ras cooperate to mediate cellular transformation by intersectin-L. J Biol Chem 2005; 280:22883 - 22891
- Aguilar RC, Longhi SA, Shaw JD, Yeh LY, Kim S, Schon A, et al. Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc Natl Acad Sci USA 2006; 103:4116 - 4121
- Coon BG, Burgner J, Camonis JH, Aguilar RC. The Epsin family of endocytic adaptors promotes fibrosarcoma migration and invasion. J Biol Chem 2010; 285:33073 - 33081
- Smith SC, Oxford G, Baras AS, Owens C, Havaleshko D, Brautigan DL, et al. Expression of Ral GTPases, their effectors and activators in human bladder cancer. Clin Canc Res 2007; 13:3803 - 3813
- Awasthi S, Singhal SS, Awasthi YC, Martin B, Woo JH, Cunningham CC, et al. RLIP76 and cancer. Clin Canc Res 2008; 14:4372 - 4377
- Goldfinger LE, Ptak C, Jeffery ED, Shabanowitz J, Hunt DF, Ginsberg MH. RLIP76 (RalBP1) is an R-Ras effector that mediates adhesion-dependent Rac activation and cell migration. J Cell Biol 2006; 174:877 - 888
- Singhal SS, Yadav S, Singhal J, Zajac E, Awasthi YC, Awasthi S. Depletion of RLIP76 sensitizes lung cancer cells to doxorubicin. Biochem Pharmacol 2005; 70:481 - 488
- Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA 2003; 100:10788 - 10793
- Santy LC, Casanova JE. Activation of ARF6 by ARNO stimulates epithelial cell migration through downstream activation of both Rac1 and phospholipase D. J Cell Biol 2001; 154:599 - 610
- Teckchandani A, Toida N, Goodchild J, Henderson C, Watts J, Wollscheid B, et al. Quantitative proteomics identifies a Dab2/integrin module regulating cell migration. J Cell Biol 2009; 186:98 - 110
- Chao WT, Kunz J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. Febs Letts 2009; 583:1337 - 1343
- Ezratty EJ, Bertaux C, Marcantonio EE, Gundersen GG. Clathrin mediates integrin endocytosis for focal adhesion disassembly in migrating cells. J Cell Biol 2009; 187:733 - 747
- Nishimura T, Kaibuchi K. Numb controls integrin endocytosis for directional cell migration with aPKC and PAR-3. Dev Cell 2007; 13:15 - 28
- Wang WD, Struhl G. Drosophila epsin mediates a select endocytic pathway that DSL ligands must enter to activate notch. Development 2004; 131:5367 - 5380
- Overstreet E, Fitch E, Fischer JA. Fat facets and liquid facets promote Delta endocytosis and Delta signaling in the signaling cells. Development 2004; 131:5355 - 5366
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 2008; 105:6392 - 6397
- Tian XL, Hansen D, Schedl T, Skeath JB. Epsin potentiates Notch pathway activity in Drosophila and C-elegans. Development 2004; 131:5807 - 5815
- Chen H, Ko G, Zatti A, Di Giacomo G, Liu LJ, Raiteri E, et al. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin1 and epsin2 in mice. Proc Natl Acad Sci USA 2009; 106:13838 - 13843
- Le Borgne R. Regulation of Notch signalling by endocytosis and endosomal sorting. Curr Opin Cell Biol 2006; 18:213 - 222
- Iwatsuki M, Mimori K, Yokobori T, Ishi H, Beppu T, Nakamori S, et al. Epithelial-mesenchymal transition in cancer development and its clinical significance. Canc Sci 2010; 101:293 - 299