Abstract
Imbalance in cytokine homeostasis plays an important part in the pathogenesis of various chronic inflammatory diseases. In multiple sclerosis (MS), the pro-inflammatory cytokine interleukin-1β (IL-1β) is present in the central nervous system, being expressed mainly in infiltrating macrophages and microglial cells. IL-1β activity is inhibited by the secreted form of IL-1 receptor antagonist (sIl-1Ra) whose production is increased in patients’ blood and induced in human monocytes by IFNβ and glatiramer acetate (GA) - both immunomodulators displaying similar therapeutic efficacy in MS. Because intracellular pathways are currently considered as potential therapeutic targets, identification of specific kinases used by both immunomodulators might lead to more specific therapeutic targeting. We addressed the question of intracellular pathways used by IFNβ and GA to induce sIL-1Ra in human monocytes in two recent studies. This addendum to these studies aims at discussing common pathways and different elements used by IFNβ and GA to induce sIL-1Ra in human monocytes. This pinpoints PI3Kδ activation as a requirement to induce sIL-1Ra production downstream monocyte stimulation by either IFNβ or GA. However, the immunomodulators differentially use MEK/ERK pathway to induce sIL-1Ra production in human monocytes. Together, our current studies suggest that PI3Kδ and MEK2 might represent new targets in MS therapy.
The secreted form of IL-1 receptor antagonist (sIL-1Ra) is a natural IL-1 inhibitor that binds IL-1 type I receptor without inducing signal transduction. Since it potently inhibits the various effects of IL-1, sIL-1Ra is considered an important regulator of the inflammatory and overall immune response mediated by IL-1, and contributes to the maintenance of cytokine homeostasis in human.Citation1–Citation3 Because it is expressed by microglial cells and infiltrating monocyte/macrophages throughout the white matter in and around the lesions, IL-1β is likely to play a part in multiple sclerosis (MS) pathogenesis.Citation4 Currently there is no cure for MS, but treatment with disease-modifying immunomodulators display beneficial effect by diminishing the severity and frequency of relapses in the relapse/remitting form of the disease. Among these drugs, IFNβ and glatiramer acetate (GA) display comparable therapeutic effects,Citation5 although their mechanisms of actions are still elusive. We made the observation that both IFNβ and GA display similar effects on the IL-1 system in vitro since they enhance the production of IL-1β induced by lipopolysaccharides (LPS) in freshly isolated human monocytes, i.e., in conditions mimicking acute inflammation, while they inhibit it in conditions mimicking chronic/sterile inflammation, i.e., T-cell contact with activated monocytes.Citation6,Citation7 Furthermore, both IFNβ and GA directly induce the production of sIL-1Ra in human monocytes.Citation7–Citation9 The latter observations corroborate the fact that sIL-1Ra levels are enhanced in the blood stream of patients treated with IFNβ and GA.Citation7,Citation10–Citation12 Since sIL-1Ra crosses the blood-brain barrier, it is likely to mediate part of the effects of IFNβ and GA within the central nervous system.
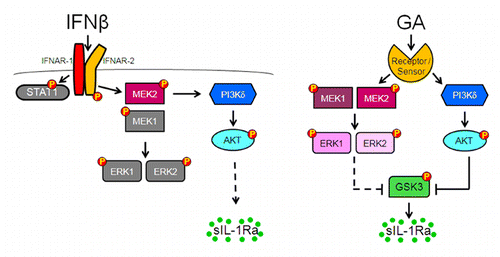
Both studies which are the subject of this addendum reveal that PI3Kδ activation is required to the triggering of sIL-1Ra production in monocytesCitation13,Citation14 confirming the key role that the kinase is playing in the optimal secretion of sIL-1Ra in monocytes independently of stimulation conditions (). Indeed, we recently reported that PI3Kδ accounts for the PI3K-dependent signaling ruling the production of sIL-1Ra in monocytes activated by LPS (acute inflammation) and contact with stimulated T cells (chronic/sterile inflammation).Citation15 Interestingly, while PI3Kδ activity is required to sIL-1Ra induction independently of the stimulus, it dampens the production of pro-inflammatory cytokines in LPS-activated monocytes, but slightly enhances it in T cell-contact-activated monocytes.Citation15 Thus, PI3Kδ is likely to be a key element in the regulation of inflammatory effector functions of human monocytes.
In contrast with PI3Kδ, although both GA and IFNβ activate the MEK/ERK pathway, the elements of the pathway are differentially required to sIL-1Ra production by human monocytes. As depicted in , the activation of MEK1 and ERK1/2 is dispensable to the production of sIL-1Ra in IFNβ-activated monocytes. Indeed, the binding of IFNβ to its specific receptor (IFNAR1-IFNAR2) induces the activation of MEK2 which is required to the activation of PI3Kδ. Both PI3Kδ and MEK2 co-localize in membrane fraction, and are essential for the activation of the PI3K/Akt pathway leading to sIL-1Ra production. Since MEK2 is recruited at membranes together with PI3Kδ, one can hypothesize that it acts as a scaffold protein as recently described by Pan et al.Citation16 Indeed, to be activated, PI3Kδ needs to interact with an YXXM motif which is absent in either IFNAR or MEK2. Our results shed light on the existence of an adaptor protein(s) and/or pathway(s) that link type I IFN receptor to MEK2 and in turn, to PI3Kδ activation. The identity of the adaptor remains to be determined. In contrast, in GA activated human monocytes, the activation of both MEK1 and MEK2 is required to optimal production of sIL-1Ra which is controlled by their downstream substrates, ERK1/2 (). Thus, when GA is recognized by a receptor (cell surface) or a sensor (inside the cell) PI3Kδ/Akt and MEK/ERK pathways are activated and are acting in parallel, being part of different signaling pathways contrasting with IFNβ signaling. Both pathways converge to phosphorylate/inactivate GSK3α/β. Since downstream elements (i.e., Akt, ERK1/2 and GSK3α/β) were phosphorylated within the same time frame it is likely that both PI3Kδ/Akt and MEK/ERK pathways are concomitantly activated to control GSK3 phosphorylation/inactivation () and in turn to regulate sIL-1Ra production in human monocytes.
To conclude, our two studies demonstrate that IFNβ and GA, both major immunomodulators used for the treatment of MS, induce sIL-1Ra production in human monocytes by triggering different crosstalks between PI3K/Akt and MEK/ERK pathways. However, at least two elements are required to the signaling of both IFNβ and GA, namely MEK2 and PI3Kδ. These shared elements might represent new molecular targets to modulate MS treatment.
Figures and Tables
Figure 1 Models of how IFNβ and GA activate PI3Kδ/Akt and MEK/ERK pathways to induce sIL-1Ra production in monocytes. (A) IFNβ binds its specific receptor (IFNAR1-IFNAR2), which induces the activation of MEK2 and the translocation of MEK2 and PI3Kδ to the membrane. The activation of PI3Kδ/Akt pathway leads to sIL-1Ra production in monocytes; Grey kinases and proteins are activated but not implicated in sIL-1Ra production. The type 1IFN canonical STAT1 pathway also is dispensable to sIL-1Ra production.Citation17 (B) GA is recognized by a receptor (cell surface) or a sensor (inside the cell) that transduces signal via activation of both PI3Kδ/Akt and MEK1/2/ERK1/2 pathways. The two pathways then converge to phosphorylate/inactivate GSK3, resulting in the induction of sIL-1Ra production. This scheme is adapted from reference Citation13.
Acknowledgements
Our work was supported by the Swiss National Science Foundation, the Swiss Society for Multiple Sclerosis and the Hans Wilsdorf Foundation. The authors are indebted to Drs. Karim J. Brandt, Nicolas Molnarfi and Patrice Lalive, and to Ms. Lyssia Gruaz, co-authors of the studies discussed in this addendum.
Addendum to: and
References
- Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis?. Best Pract Res Clin Rheumatol 2006; 20:879 - 896
- Arend WP, Palmer G, Gabay C. IL-1, IL-18 and IL-33 families of cytokines. Immunol Rev 2008; 223:20 - 38
- Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the inter-leukin-1-receptor antagonist. N Engl J Med 2009; 360:2426 - 2437
- Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol 1995; 37:424 - 435
- Mikol DD, Barkhof F, Chang P, Coyle PK, Jeffery DR, Schwid SR, et al. Comparison of subcutaneous interferon beta-1a with glatiramer acetate in patients with relapsing multiple sclerosis (the REbif vs. Glatiramer Acetate in Relapsing MS Disease [REGARD] study): a multicentre, randomised, parallel, open-label trial. Lancet Neurol 2008; 7:903 - 914
- Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite effects of IFNbeta on cytokine homeostasis in LPS- and T cell contact-activated human monocytes. J Neuroimmunol 2004; 146:76 - 83
- Burger D, Molnarfi N, Weber MS, Brandt KJ, Benkhoucha M, Gruaz L, et al. Glatiramer acetate increases IL-1 receptor antagonist but decreases T cell-induced IL-1beta in human monocytes and multiple sclerosis. Proc Natl Acad Sci USA 2009; 106:4355 - 4359
- Coclet-Ninin J, Dayer JM, Burger D. Interferon-beta not only inhibits interleukin-1beta and tumor necrosis factor-alpha but stimulates interleukin-1 receptor antagonist production in human peripheral blood mononuclear cells. Eur Cytokine Netw 1997; 8:345 - 349
- Jungo F, Dayer JM, Modoux C, Hyka N, Burger D. IFNbeta inhibits the ability of T lymphocytes to induce TNFalpha and IL-1beta production in monocytes upon direct cell-cell contact. Cytokine 2001; 14:272 - 282
- Nicoletti F, Patti F, DiMarco R, Zaccone P, Nicoletti A, Meroni P, et al. Circulating serum levels of IL-1ra in patients with relapsing remitting multiple sclerosis are normal during remission phases but significantly increased either during exacerbations or in response to IFNbeta treatment. Cytokine 1996; 8:395 - 400
- Perini P, Tiberio M, Sivieri S, Facchinetti A, Biasi G, Gallo P. Interleukin-1 receptor antagonist, soluble tumor necrosis factor-alpha receptor type I and II and soluble E-selectin serum levels in multiple sclerosis patients receiving weekly intramuscular injections of interferon-beta1a. Eur Cytokine Netw 2000; 11:81 - 86
- Comabella M, Julia E, Tintore M, Brieva L, Tellez N, Rio J, et al. Induction of serum soluble tumor necrosis factor receptor II (sTNF-RII) and interleukin-1 receptor antagonist (IL-1ra) by interferon beta-1b in patients with progressive multiple sclerosis. J Neurol 2008; 255:1136 - 1141
- Carpintero R, Brandt KJ, Gruaz L, Molnarfi N, Lalive PH, Burger D. Glatiramer acetate triggers PI3K{delta}/Akt and MEK/ERK pathways to induce IL-1 receptor antagonist in human monocytes. Proc Natl Acad Sci USA 2010; 107:17692 - 17697
- Brandt KJ, Carpintero R, Gruaz L, Molnarfi N, Burger D. A novel MEK2/PI3K{delta} pathway controls the expression of IL-1 receptor antagonist in IFN{beta}-activated human monocytes. J Leukoc Biol 2010; 88:1191 - 1200
- Molnarfi N, Brandt KJ, Gruaz L, Dayer JM, Burger D. Differential regulation of cytokine production by PI3Kδelta in human monocytes upon acute and chronic inflammatory conditions. Mol Immunol 2008; 45:3419 - 3427
- Pan CQ, Liou YC, Low BC. Active Mek2 as a regulatory scaffold that promotes Pin1 binding to BPGAP1 to suppress BPGAP1-induced acute Erk activation and cell migration. J Cell Sci 2010; 123:903 - 916
- Molnarfi N, Hyka-Nouspikel N, Gruaz L, Dayer JM, Burger D. The production of IL-1 receptor antagonist in IFN-{beta}-stimulated human monocytes depends on the activation of phosphatidylinositol 3-kinase but not of STAT1. J Immunol 2005; 174:2974 - 2980