Abstract
The dynamin proteins have been associated with the process of endocytosis for many years. Until recently it was considered that yeast dynamin-related proteins did not play a role in endocytosis and the proposed scission function of dynamin was attributed to another group of proteins, the amphiphysins. However, it has now been shown that the yeast dynamin-like protein Vps1 shows a transient burst of localization to sites of endocytosis. Vps1 assembles at cortical sites at the time when actin polymerization is proposed to drive plasma membrane invagination. In concert with the amphiphysins Vps1 is then thought to function in the scission step to release a formed vesicle. It was shown that a mutation preventing self assembly of Vps1 caused a defect in endocytosis but not in other functions with which Vps1 is associated. Using electron microscopy we now show that this mutation I649K, corresponding to I690K in human Dyn1, causes formation of long endocytic invaginations. The data suggest that an ability of Vps1 to self assemble and to thereby stimulate its GTPase activity is critical for the 'pinching-off' stage of endocytosis to form a vesicle.
Vps1 is a dynamin-related protein that has an N-terminal GTPase activity, a middle domain and a C-terminal GTPase effector domain (GED). In dynamins this latter domain is also proposed to function in self assembly of the protein into oligomeric ring or spiral structures. Unlike classical dynamins such as dynamin-1 or -2 (Dyn1, Dyn2) in mammals, Vps1 does not have a C-terminal proline-arginine rich domain or a PH domain, but it is still able to bind lipids.Citation1 Vps1 has long been recognised to function in several membrane trafficking pathways including Golgi and vacuolar protein sorting, and also in peroxisomal fission.Citation2–Citation4 Possibly because of these multiple roles, or because of technical difficulties in visualising the protein using fluorescent protein tags, a function of Vps1 in endocytosis, similar to that of Dyn1 or Dyn2 was generally considered unlikely. However, the recent demonstration of Vps1 at endocytic sites and further analysis of Vps1 mutants now gives strong support for a role of this dynamin protein in the endocytic process.Citation1 Vps1 arrives at the endocytic site just at the time the membrane begins to invaginate. It disassembles at the point of scission and it appears to function with the amphiphysin heterodimer Rvs161/Rvs167 to facilitate the vesicle scission step.Citation1 Interestingly, the ultrastructural analysis from electron microscopy studies indicated that in the absence of vps1 the tubules invaginated aberrantly and were formed at a broader range of angles from the plasma membrane. This suggested that Vps1 plays a role prior to scission in ensuring directed invagination of the membrane.Citation1 In vivo studies of Vps1 mutants indicated that both the N-terminal GTPase domain and the C-terminal GTPase Effector domain (GED) involved in self-assembly, play a role in endocytosis.Citation1,Citation5
In Dyn1 it is proposed that the GED domain acts as an intramolecular GTPase activator protein (GAP) and that GTP hydrolysis causes the displacement of dynamin from the membrane surface.Citation6,Citation7 If this is also the case for Vps1 in S. cerevisiae then a mutant that cannot activate its GTPase due to a self-assembly defect might be predicted to accumulate at the membrane in an active (GTP-bound) state. This would be a distinct situation from the absence of Vps1 when there would be no active GTP-bound Vps1 at the endocytic site. Analysis of cells expressing the self assembly mutant I649K might then reveal insights into the function of the GTP bound form of Vps1 at the endocytic site.
Cells deleted for VPS1 were transformed with plasmids expressing wild-type VPS1, Vps1I649K or an empty plasmid. These were grown and processed for electron microscopy as previously described in reference Citation1. As shown in there is a marked change in the cells expressing the I649K mutant. Rather than invaginations that are restricted to a length of about 100 nm, these cells now have many invaginations that are more than twice this length. These invaginations can be directed straight into the cell or, more frequently they appear to bend back up toward the plasma membrane, especially as they get longer. Invaginations of this kind are not seen in wild-type or in vps1Δ cells. This demonstrates that the presence of a Vps1 mutant that cannot self-assemble, and therefore cannot stimulate its GTPase activity, inhibits the normal scission process and that this only happens when the N-terminal domain of Vps1 is present.
Taking account of this, and other data for Vps1, we consider that Vps1 plays an important role in endocytosis in yeast as outlined in . It is recruited at the onset of invagination and appears to play a role in directing the formation of the invaginating tubule. However, when the mutant Vps1 is expressed that cannot self-assemble (I649K) we observe extended invaginations that suggest tubulation factors in the cells can be recruited in this state but that scission itself cannot progress. Interestingly Rvs167 does not have a longer lifetime in this mutantCitation1 indicating that the increased level of invagination is not due to uncontrolled amphiphysin activity. Furthermore, the in vitro analysis of I649K indicates that it alone cannot tubulate membranes. The increased tubulation therefore seems likely to be a function of the presence of GTP bound Vps1 at the endocytic site, but another protein(s) is responsible for the increase in invagination length.
Currently there are several models for how dynamins might function in scission. These include it acting in a mechanochemical role in driving scission by functioning as a ‘pinchase’ activity; in a more regulatory role where in the GTP bound form the dynamin recruits effectors that mediate vesicle formation, and also that it may function to protect the PtdIns(4,5)P2 rich part of the invaginated membrane leading to a lipid boundary which itself weakens the membrane and leads to scission.Citation8,Citation9 It is also possible that all three of these proposed functions may contribute to the final scission event. We consider that the data shown here provides evidence that the transition from the GTP-bound state to the GDP-bound state, or the conformational change involved in eliciting this change, plays a role in allowing the fission step to proceed and provides further strong evidence for a direct role of Vps1 in yeast endocytosis. Further studies on Vps1 combining ultrastructural analysis with live cell imaging and biochemical approaches, with a range of mutants, might now allow us to use this model system to more specifically address the questions that still remain concerning molecular mechanism of dynamin function in endocytosis.
Figures and Tables
Figure 1 Transmission electron micrographs of endocytic invaginations in wild type, vps1Δ and Vps1 I649K expressing cells. Transmission electron micrographs of 40–60 nm ultrathin sections of high pressure frozen/freeze substituted whole yeast cells showing representative invaginations of the plasma membrane in cells expressing wild type vps1 (a), no vps1 (b) or expressing the I649K vps1 mutant (c). In wild type and vps1 deletion strains most invaginations vary in depth between 20–60 nm and they are never more than 100 nm. In the I649K mutant, many invaginations are in this normal range, but a significant number exceed 100 nm (c) and can be over 300 nm in depth.
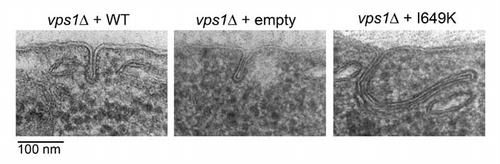
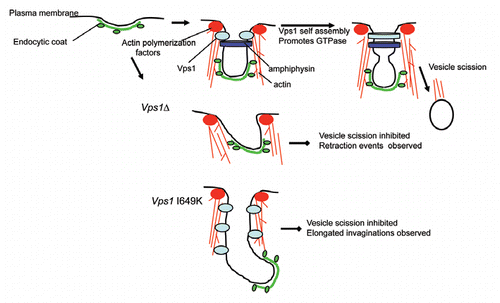
Figure 2 Schematic model of Vps1 function in endocytosis. During endocytic invagination in wild type cells Vps1 arrives at the onset of invagination and remains at the site until scisson occurs. In the absence of Vps1, the amphiphysin heterodimer Rvs161/Rvs167 localizes less well to the site and many events appear to retract towards the plasma membrane and the invaginations fail to undergo scission. Invaginations are also less likely to be directed perpendicularly into the cell. However in the presence of a self assembly defective mutant, vps1 I649K, the intramolecular GTPase cannot be stimulated and elongated invaginations are observed. This suggests that the transition of Vps1 to the self assembled form is part of the mechanism involved in vesicle scission.
Acknowledgements
The work was supported by a MRC Senior non-clinical fellowship to K.R.A. (G0601600) and Biotechnology and Biological Sciences Research Council grant supporting I.S. (BB/G011001/1). R.M. and M.W.G. are supported by a grant from the Biotechnology and Biological Sciences Research Council, UK, grant number BB/G011818/1.
Addendum to:
References
- Smaczynska-de Rooij II, Allwood EG, Aghamohammadzadeh S, Hettema EH, Goldberg MW, Ayscough KR. A role for the dynamin-like protein Vps1 during endocytosis in yeast. J Cell Sci 2010; 123:3496 - 3506
- Hoepfner D, van den Berg M, Philippsen P, Tabak HF, Hettema EH. A role for Vps1p, actin and the Myo2p motor in peroxisome abundance and inheritance in Saccharomyces cerevisiae. J Cell Biol 2001; 155:979 - 990
- Peters C, Baars TL, Buhler S, Mayer A. Mutual control of membrane fission and fusion proteins. Cell 2004; 119:667 - 678
- Vater CA, Raymond CK, Ekena K, Howaldstevenson I, Stevens TH. The Vps1 protein, a homolog of dynamin required for vacuolar protein sorting in Saccharomyces cerevisiae, is a GTPase with 2 functionally separable domains. J Cell Biol 1992; 119:773 - 786
- Nannapaneni S, Wang D, Jain S, Schroeder B, Highfill C, Reustle L, et al. The yeast dynamin-like protein Vps1:vps1 mutations perturb the internalization and the motility of endocytic vesicles and endosomes via disorganization of the actin cytoskeleton. Eur J Cell Biol 89:499 - 508
- Danino D, Moon KH, Hinshaw JE. Rapid constriction of lipid bilayers by the mechanochemical enzyme dynamin. J Struct Biol 2004; 147:259 - 267
- Ramachandran R, Schmid SL. Real-time detection reveals that effectors couple dynamin's GTP-dependent conformational changes to the membrane. EMBO J 2008; 27:27 - 37
- Liu J, Sun YD, Oster GF, Drubin DG. Mechanochemical crosstalk during endocytic vesicle formation. Curr Opin Cell Biol 22:36 - 43
- Mettlen M, Pucadyil T, Ramachandran R, Schmid SL. Dissecting dynamin's role in clathrin-mediated endocytosis. Biochem Soc Transact 2009; 37:1022 - 1026