Abstract
Early embryos of many vertebrates and invertebrates develop outside the mother and are exposed to myriads of potential microbial colonizers. Here we discuss how these embryos are protected from microbial attacks and how they might control and shape their microbiota. In essence we delineate a new role for antimicrobial peptides both in selecting particular bacterial partners during early development and in being important components of a “be prepared” strategy providing transgenerational protection.
Introduction
“Considering the importance of the fetus to our survival as a species, it is surprising that we know so little about what protects it from microbial assault.”Citation1 Mammalian embryos are embedded in the uterus, which provides protection during embryogenesis. Via the placenta, the mother provides oxygen and nutrients as well as embryo protection by transferring antibodies across the placenta to allow humoral immune responses early in life. Postnatally, mammals transfer antibodies via breast milk to the offspring supporting the neonate immune system.Citation2 Birds,Citation3 fish,Citation4,Citation5 amphibiansCitation6 and reptilesCitation7 transmit passive immunity through the deposition of antibodies in eggs. In contrast to mammalian vertebrates, many other vertebrates and invertebrates release their oocytes in an environment full of microbes to develop there as “orphan” embryos. How these seemingly unprotected embryos respond to the environment-specific microbial challenge is an interesting albeit not yet understood problem. The most critical phase in the development of any embryo appears to be the period prior to maternal-zygotic transition (MZT) when the embryo starts to utilize its own transcriptional machinery.Citation8,Citation9 In this period the cells do not transcribe their own genes as they have only a biphasic cell cycle consisting of only two steps: the M and the S phase. Only after the MZT, when G1 and G2 phases are added to the cell cycle, embryos are able to respond actively to environmental signals for example with production of stress proteins.Citation8 Hence, how is bacterial colonization of the early embryo controlled before MZT?
Maternal AMPs Protect the Embryo: The “Be Prepared” Strategy
For a long time now, there has been a growing awareness of vertebrate developmental biologists for the significance of the so-called “fertilization envelope” in providing microbial protection in early developmental stages. In fish, for example, the fertilization envelope shows both bactericidal activity against Vibrio anguillarumCitation10 and antifungal action against Saprolegnia parasitica.Citation11 This protection has to be achieved by maternal mechanisms as the early fish embryo is not using its own transcriptional machinery before MZT. Similarly, the extra-embryonic tissue of invertebrates such as the tobacco hornworm is immune competent and most likely protects the embryo from infection.Citation12 In bumblebees, freshly laid eggs exhibit a strong antibacterial activity which is significantly increased after maternal challenge.Citation13,Citation14 In social insects where potential pathogens faced by the mother are also an immediate threat to the offspring this priming is of special relevance.Citation14
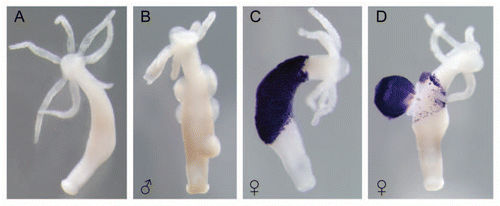
While in most invertebrates the nature of the molecules involved in maternal protection are not known yet, the freshwater polyp Hydra uses maternally-encoded antimicrobial peptides of the periculin familyCitation15 to protect its embryos. In female Hydra, oocytes differentiate from clusters of interstitial stem cells committed to the female germlineCitation16–Citation19 and develop into a mature egg which is attached outside the mother ( and D). Upon commitment to female gametogenesis, female interstitial cells, often referred to as nurse cells, produce AMPs of the peptide family periculin and store it in vesicles.Citation15 Within each cluster of interstitial cells, one of the cells develops into an oocyte, while the other nurse cells are phagocytosed and become incorporated into the cytoplasm of the developing oocyte.Citation16–Citation18 Condensed nurse cells constitute the bulk of the ooplasm, persist throughout embryogenesis and provide active AMPs including members of the periculin family for the developing oocyte. Following fertilization, periculin containing vesicles get released to the surface of the developing embryo.
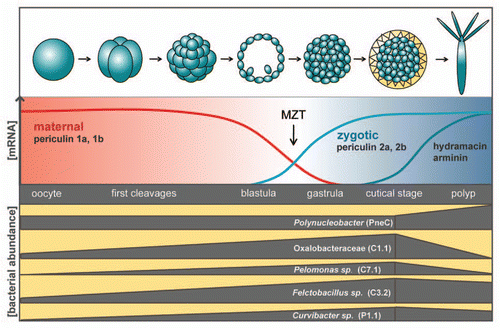
During embryogenesis the number of bacterial colonizers is increasing in number and changing in composition. For example, the bacterial phylotypes C7.1, belonging to the Pelomonas group and P1.1, representing Curvibacter sp. are present only in late developmental stages while they appear to be absent in the early embryoCitation15 (). Thus, early developmental stages appear to have a microbiota which is clearly distinct from later developmental stages. Interestingly, the differential colonization is reflected in differences in antimicrobial activity in embryos compared to adult polyps.Citation15 Beginning with the gastrula stage, i.e., after MZT, Hydra embryos express a set of periculin peptides (periculin 2a and 2b) which replaces the maternal produced periculin peptides 1a and 1b (). This shift in the expression within the periculin peptide family represents a shift from maternal to zygotic protection of the embryo. In adult Hydra polyps, additional AMPs including hydramacinCitation20 and arminin,Citation21 contribute to the host-derived control of bacterial colonization ().
Antimicrobial Peptides are Regulators Rather than Killers: An Evolutionary Perspective
Antimicrobial peptides usually are considered as an effective innate immune weapon against microbial intruders. This view, however, may be a bit simplistic since ectopic overexpression of antimicrobial periculin1a peptide in, for example, hydra's ectodermal epithelial cells does not cause simple disappearance of the associated microbes but results in a distinct change in the composition of the microbiota.Citation15 Testing the hypothesis of antimicrobial peptides being capable of causing changes in the microbial composition in mice, Salzmann et al.Citation22,Citation23 recently used a transgenic approach and observed that mice expressing human alpha-defensin-5 (DEFA5) and mice lacking an enzyme required for the processing of mouse alpha-defensins show significant changes in intestinal microbiota composition, but not in total bacterial numbers. They conclude that defensins play a homeostatic role in regulating the makeup of the commensal microbiota. This is consistent with the observations made in the Hydra model, where maternal AMPs maintain the homeostasis between the bacterial colonizers and the epithelium of the early development stages.Citation15 These and other observations make it likely, that AMPs function as host-derived regulators of microbial colonization rather than as simple killers.
When considering host microbe interactions from an evolutionary perspective, the diversity of microbes colonizing a given host is a result of coevolution between the host and the associated microbial community. Gordon and colleaguesCitation24 suggested earlier that the specific structure of the microbial community associated with a given host is a result of natural selection at two levels. First, competition between members of the microbiota would exert “bottom-up” selection and second, the host level would represent a “top-down” selection on the microbial community.Citation24 The innate immune system is the hosts' first line of contact with the microbiota and probably plays a crucial role in this “top-down” selection of the microbiota. Indirect evidence supporting this view comes mainly from the observation that defects in the host innate defense system affect bacterial colonization of the intestine. Crohn's disease and ulcerative colitis patients, for example, have abnormal composition of gastrointestinal microbes, characterized by the depletion of members of the phyla Firmicutes and Bacteroidetes,Citation25 two bacterial divisions dominating the distal gut microbiota.Citation26,Citation27 Interestingly, patients with Crohn's disease show also a reduced antibacterial activity in their intestinal mucosal extracts with strongly reduced expression of paneth cell α-defensins compared to control group of patients.Citation28
When considering the function of antimicrobial peptides from an evolutionary perspective, it may be relevant to consider that most if not all AMPs are restricted to a specific genus or even a speciesCitation29 and are representing so-called taxonomically-restricted genes (TRGs).Citation30 We have proposed elsewhere, that this may reflect habitat-specific adaptations to control habitat-specific microbial colonizers.Citation30 Evolutionary changes in the AMP repertoire of host species would therefore lead to changes in the composition of the associated bacterial community. Since the genetic information encoded by microorganisms can change under environmental demands more rapidly than the genetic information encoded by the host organism, Rosenberg and colleagues suggested in their hologenome theory,Citation31 that changed microbial partners confer greater adaptive potential to environmental changes than alteration and selection processes required for host genome evolution alone.
Role of Associated Bacteria in Embryo Protection
Why Hydra embryos appear to select developmental stage-specific microbes is not yet understood. Are the associated beneficial microbes involved in embryo protection? Observations in a number of aquatic animals indicate a protective function of associated symbionts for the early embryo. For example, embryos of the crustacean species Palaemon macrodactylus are colonized by the symbiotic bacteria Alteromonas sp. producing the secondary metabolite 2,3-indolinedione which is active against a pathogenic fungus.Citation32 Bacteria-free embryos get quickly infected and die, whereas embryos reinoculated with Aeromonas sp. or treated with with 2,3-indolinedione persist the infection.Citation32 Similarly, in the salamander Hemidactylium scutatum a protective antifungal molecules derived from the associated bacterial community resides in the skin.Citation33 One member of this community, the bacterial symbiont Janthinobacterium lividum produces two metabolites, indole-3-carboxaldehyde and violacein, which protect against infection with the fungus Batrachochytrium dendrobatidis.Citation34 In addition to adult salamanders, these bacteria may protect also eggs and embryos from fungal infection since they can be passed directly from the mother to offspring in each generation and since communal nesting increases the likelihood of the transmission of the protective bacteria to the eggs.Citation35 Preliminary observations in Hydra also point to a role associated bacteria in microbial defense since bacteria-free polyps and embryos are prone to severe fungal infection, while control animals show no evidence of fungal growth (Franzenburg & Fraune S, personal observation).
Conclusions
We have discussed elsewhere the role of bacteria in animal development.Citation36 Here we show that animal hosts at different developmental stages select for specific microbes by using distinct sets of antimicrobial peptides and that these beneficial stage-specific bacteria can have essential roles in antibacterial and antifungal defenses. However, despite the obvious and proven importance of interactions between microbes and their hosts and the fact that hosts control microbial community composition by antimicrobial peptides, little is known at present about the rules that govern the host-microbe assemblies. What are the contributions of species interactions? Is there selection at the community level, and if so, how? Equally pressing questions concern the stability and robustness of within-host microbial communities. A key point is to understand how far the overall function of the microbiota is influenced by individual as well as synergistic contributions of community members. An in-depth understanding of these points requires systematic phenotypic screens in combination with an analysis of the underlying molecular interactions, taking into account the relevant environmental variables.
Figures and Tables
Acknowledgements
This work was supported in part by German Research Foundation (DFG) Grants (to T.C.G.B.) and German Research Foundation (DFG) Cluster of Excellence programs “The Future Ocean” and “Inflammation at Interfaces”.
References
- Zasloff M. Vernix, the newborn and innate defense. Pediatr Res 2003; 53:203 - 204
- Boulinier T, Staszewski V. Maternal transfer of antibodies: raising immuno-ecology issues. Trends Ecol Evol 2008; 23:282 - 288
- Grindstaff JL, Hasselquist D, Nilsson JK, Sandell M, Smith HG, Stjernman M. Transgenerational priming of immunity: maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc Biol Sci 2006; 273:2551 - 2557
- Bly JE, Grimm AS, Morris IG. Transfer of passive immunity from mother to young in a teleost fish: haemagglutinating activity in the serum and eggs of plaice, Pleuronectes platessa L. Comp Biochem Physiol A Comp Physiol 1986; 84:309 - 313
- Fuda H, Hara A, Yamazaki F, Kobayashi K. A peculiar immunoglobulin M (IgM) identified in eggs of chum salmon (Oncorhynchus keta). Dev Comp Immunol 1992; 16:415 - 423
- Poorten TJ, Kuhn RE. Maternal transfer of antibodies to eggs in Xenopus laevis. Dev Comp Immunol 2009; 33:171 - 175
- Schumacher IM, Rostal DC, Yates RA, Brown DR, Jacobson ER, Klein PA. Persistence of maternal antibodies against Mycoplasma agassizii in desert tortoise hatchlings. Am J Vet Res 1999; 60:826 - 831
- Newport J, Kirschner M. A major developmental transition in early Xenopus embryos: I. characterization and timing of cellular changes at the midblastula stage. Cell 1982; 30:675 - 686
- Tadros W, Lipshitz HD. The maternal-to-zygotic transition: a play in two acts. Development 2009; 136:3033 - 3042
- Kudo S, Inoue M. Bacterial action of fertilization envelope extract from eggs of the fish Cyprinus carpio and Plecoglossus altivelis. J Exp Zool 1989; 250:219 - 228
- Kudo S, Teshima C. Enzyme-activities and antifungal action of fertilization envelope extract from fish eggs. J Exp Zool 1991; 259:392 - 398
- Gorman MJ, Kankanala P, Kanost MR. Bacterial challenge stimulates innate immune responses in extra-embryonic tissues of tobacco hornworm eggs. Insect Mol Biol 2004; 13:19 - 24
- Moret Y, Schmid-Hempel P. Immune defence in bumble-bee offspring. Nature 2001; 414:506
- Sadd BM, Schmid-Hempel P. Facultative but persistent trans-generational immunity via the mother's eggs in bumblebees. Curr Biol 2007; 17:1046 - 1047
- Fraune S, Augustin R, Anton-Erxleben F, Wittlieb J, Gelhaus C, Klimovich VB, et al. In an early branching metazoan, bacterial colonization of the embryo is controlled by maternal antimicrobial peptides. Proc Natl Acad Sci USA 2010; 107:18067 - 18072
- Honegger TG, Zurrer D, Tardent P. Oogenesis in Hydra carnea: A new model based on light and electron microscopic analyses of oocyte and nurse cell differentiation. Tissue Cell 1989; 21:381 - 393
- Martin VJ, Littlefield CL, Archer WE, Bode HR. Embryogenesis in Hydra. Biol Bull 1997; 192:345 - 363
- Tardent P. Gametogenesis, fertilization and embryogenesis—Introductory remarks. Amer Zool 1974; 14:443 - 445
- Tannreuther GW. The development of Hydra. Biol Bull 1908; 14:261 - 281
- Bosch TC, Augustin R, Anton-Erxleben F, Fraune S, Hemmrich G, Zill H, et al. Uncovering the evolutionary history of innate immunity: the simple metazoan Hydra uses epithelial cells for host defence. Dev Comp Immunol 2009; 33:559 - 569
- Augustin R, Anton-Erxleben F, Jungnickel S, Hemmrich G, Spudy B, Podschun R, et al. Activity of the novel peptide arminin against multiresistant human pathogens shows the considerable potential of phylogenetically ancient organisms as drug sources. Antimicrob Agents Chemother 2009; 53:5245 - 5250
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010; 11:76 - 83
- Salzman NH, Underwood MA, Bevins CL. Paneth cells, defensins and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin Immunol 2007; 19:70 - 83
- Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell 2006; 124:837 - 848
- Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 2007; 104:13780 - 13785
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444:1022 - 1023
- Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA 2004; 101:4596 - 4601
- Wehkamp J, Salzman NH, Porter E, Nuding S, Weichenthal M, Petras RE, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci USA 2005; 102:18129 - 18134
- Wang Z, Wang G. APD: the Antimicrobial Peptide Database. Nucleic Acids Res 2004; 32:590 - 592
- Khalturin K, Hemmrich G, Fraune S, Augustin R, Bosch TCG. More than just orphans: are taxonomically-restricted genes important in evolution?. Trends Genet 2009; 25:404 - 413
- Rosenberg E, Koren O, Reshef L, Efrony R, Zilber-Rosenberg I. The role of microorganisms in coral health, disease and evolution. Nat Rev Microbiol 2007; 5:355 - 362
- Gil-Turnes MS, Hay ME, Fenical W. Symbiotic marine bacteria chemically defend crustacean embryos from a pathogenic fungus. Science 1989; 246:116 - 118
- Becker MH, Harris RN. Cutaneous bacteria of the redback salamander prevent morbidity associated with a lethal disease. PloS One 2010; 5:10957 - 1
- Brucker RM, Harris RN, Schwantes CR, Gallaher TN, Flaherty DC, Lam BA, et al. Amphibian chemical defense: antifungal metabolites of the microsymbiont Janthinobacterium lividum on the salamander Plethodon cinereus. J Chem Ecol 2008; 34:1422 - 1429
- Banning JL, Weddle AL, Wahl GW III, Simon MA, Lauer A, Walters RL, et al. Antifungal skin bacteria, embryonic survival and communal nesting in four-toed salamanders, Hemidactylium scutatum. Oecologia 2008; 156:423 - 429
- Fraune S, Bosch TC. Why bacteria matter in animal development and evolution. Bioessays 2010; 32:571 - 580