Abstract
Pattern recognition receptors (PRRs) constitute the first line of host defense against bacterial, fungal and viral pathogens. Upon sensing microbial infection, PRRs initiate a cascade of signal transduction and transcriptional events to induce the production of inflammatory cytokines. As a result, many pathogens have evolved to evade PRR detection and activation in order to establish a successful infection. In a recent report, we described how a viral protein named Orf63 encoded by Kaposi’s sarcoma-associated herpesvirus (KSHV) inhibits activation of several members of a family of PRRs called NLRs (nucleotide-binding and oligomerization, leucine-rich repeat) by functionally inhibiting the NLR response. This resulted in reduced NLR-dependent pro-inflammatory cytokine secretion and cell death. Moreover, Orf63 was essential in the KSHV lifecycle. Thus, our work suggests KSHV has evolved to encode a functional homolog of NLR proteins in an effort to suppress the host inflammatory response.
Kaposi's sarcoma-associated herpesvirus is the etiological agent of the leading acquired immune deficiency syndrome (AIDS)-defining tumor, Kaposi's sarcoma (KS).Citation1 KSHV is also associated with two additional cancers, primary effusion lymphoma (PEL) and multicentric Castleman's disease (MCD). Like all herpesviruses, KSHV establishes a life-long persistent infection, which is dependent on its ability to block the host's anti-viral immune response. Although many KSHV pathogenic determinants have been discovered, the functions of many virus-encoded proteins remain uncharacterized.
A component of the host anti-pathogen response that is of emerging importance is the NLR family of proteins. NLRs are members of a broader class of pathogen sensing molecules called pattern recognition receptors (PRR), which drive immune cell activation in response to bacterial or viral infection.Citation2 There is increasing evidence for the association of NLR family members with human metabolic disorders, cancer development and treatment as well as autoimmune disorders.Citation3 NLRs mediate antiviral immune responses to RNA and DNA viruses such as Influenza, Vaccinia and other highly pathogenic viruses.Citation4 Several NLRs form large molecular structures called inflammasomes, involving multiple NLR molecules, apoptotic-associated speck-like protein (ASC) and procaspase-1. Inflammasome formation results in the subsequent autocatalytic processing of procaspase-1 to caspase-1, which is required for caspase-1-dependent processing of proinflammatory cytokines, pro- IL-1β and pro-IL-18 to their biologically active forms, IL-1β and IL-18, respectively. IL-1β and IL-18 are anti-viral cytokines and caspase-1 activation results in an inflammatory cell death process termed pyroptosis.Citation5–Citation7 Recently, NLRs also have been found to modulate expression of other antiviral cytokines and chemokines during viral infection. Thus, NLRs are a key component of the host defense mechanism against invading pathogens, and their role in combating infection and their contribution to human disease is only just being understood.
Viruses have evolved to evade the host immune response by encoding proteins that prevent inflammasome signaling. For example, several poxviruses express a protein, pyrin-only protein (POP), that blocks the association of ASC with a given NLR through POP's interaction with ASC.Citation8 Furthermore, infection with a POP-deficient virus showed increased IL-1β and IL-18 secretion. Additional viral inhibitors of IL-1β and IL-18 secretion include the poxvirus caspase-1 inhibitor CrmA and the Influenza A virus protein NS1.Citation9,Citation10 Given that NLRs play a significant role in modulating viral infection, it is likely that additional NLR-inhibiting viral proteins exist.
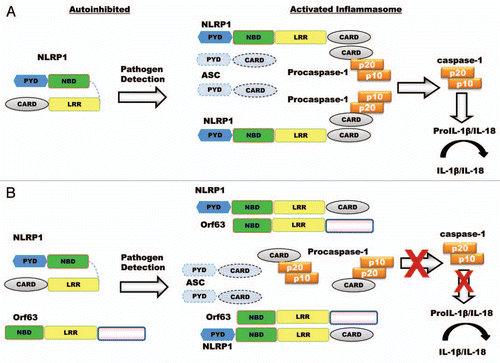
In our recent report, we characterized a novel KSHV tegument protein, Orf63, which showed sequence similarity to the NLR family member NLRP1.Citation11 Biochemical analyses demonstrated that Orf63 directly interacted with NLRP1, suggesting Orf63 disrupted NLRP1's association with ASC or procaspase-1. Indeed, we observed that Orf63 blocked the ability of NLRP1 to associate with procaspase-1, and subsequently inhibited procaspase-1 processing and secretion of IL-1β and IL-18 (). NLRP1 can function independently of ASC. Thus, it is logical that Orf63 would target the necessary interaction between procaspase-1 with NLRP1.
Surprisingly, we found that Orf63 also interacted with two additional NLR family members, NOD2 and NLRP3. Orf63 only showed sequence similarity to the nucleotide binding (NBD) and leucine rich repeat (LRR) domains of NLRP1, which are domains conserved across all NLR family members. It has been demonstrated that in the inactivated state, the LRR will fold back onto the NBD to inhibit NLR activation.Citation12 This suggests that Orf63 might mimic this interaction to inhibit NLRP1 and potentially other NLRs. Further work is necessary to elucidate the minimal molecular elements required for interaction between Orf63 and NLRs.
To demonstrate whether Orf63 was important in the KSHV lifecycle, we showed that knockdown of Orf63 expression by small-interfering RNA (siRNA) during KSHV infection of human monocytes resulted in increased secretion of IL-1β. In addition, siRNA knockdown of Orf63 during KSHV reactivation from latency in PEL, a tumor cell line that harbors latent KSHV, decreased infectious virus release. This indicates that Orf63 is critical for blocking NLR responses to both the primary infection and reactivation phases of the KSHV lifecycle. Herpesvirus tegument proteins are perfectly positioned during viral entry since they are released from the virion into the cytosol during infection.Citation13,Citation14 Since Orf63 is present in the tegument, KSHV is able to release this NLR inhibitor into the cell at the onset of primary infection, thereby enabling inhibition of host immunity and productive infection.
Whether NLRs mediate the host response to KSHV infection was previously unknown. Therefore, we investigated the effect of NLRP1 activation on KSHV reactivation from latency. In a NLRP1 inflammasome reconstitution model, we demonstrated that NLRP1 blocked KSHV's ability to replicate upon induced reactivation. The specificity for NLRP1 was confirmed by co-expressing the LRR of NLRP1, an inhibitor of full-length NLRP1, which subsequently restored reactivation. NLRP1's ability to modulate reactivation was confirmed in PEL cells, in which siRNA knockdown of NLRP1 resulted in increased viral replication and production of infectious virus. These data underscore the need for further exploration into the relationship between NLRs and their role in herpesvirus biology.
KS is the major AIDS-associated tumor and our report highlights the need to better understand how KSHV infects and establishes itself in the human host. Our findings suggest that KSHV has specifically evolved to encode a functional homolog of cellular NLRP1 and evokes the possibility that other viruses encode similar proteins. KSHV Orf63 is the first pathogen-encoded inhibitor of IL-1β that directly interacts with an NLR. Targeting viral inhibitors of NLR family members presents possible therapeutic strategies in the treatment of viral diseases. Given the current number of inflammatory diseases, the potential to harness Orf63′s properties for future therapeutics that target inflammation could prove valuable for reducing the severity of inflammation-associated disorders.
Abbreviations
| KSHV/HHV8 | = | kaposi's sarcoma-associated herpesvirus/human herpesvirus 8 |
| PRR | = | pattern recognition receptor |
| NLR | = | nucleotide binding and oligomerization, leucine rich repeat |
| AIDS | = | acquired-immune deficiency syndrome |
| PEL | = | primary effusion lymphoma |
| MCD | = | multicentric Castleman's disease |
| ASC | = | apoptotic-associated speck-like protein |
| POP | = | pyrin-only protein |
| IL-1β/IL-18 | = | interleukin-1β/18 |
Figures and Tables
Figure 1 The effect of Orf63 on the formation and activity of the NLRP1 inflammasome. (A) Normal activation of the NLRP1 inflammasome. NLRP1 exists in an autoinhibited state in the absence of pathogen infection where the LRR domain folds back onto the NBD to prevent inflammasome formation. Pathogen detection induces NLRP1 into an open conformation facilitating the association with procaspase-1 through homotypic interactions between CARD domains to form the inflammasome. The association of NLRP1 with procaspase-1 initiates autocatalytic processing of procaspase-1 to catalytically active caspase-1, which cleaves pro-IL-1β and/or IL-18 to their biologically active forms. (B) Orf63 disrupts the interaction of procaspase-1 and NLRP1. Through molecular mimicry of NLRP1's NBD and LRR, Orf63 interacts directly with NLRP1 and blocks NLRP1 inflammasome formation preventing downstream procaspase-1 activation and subsequent IL-1β/IL-18 processing.
Acknowledgments
We thank Stefanie Mach and John West for critical reading of the manuscript. B.D. is supported by NIH grants CA096500, DE018281, American Heart Association grant 0640041N and a Burroughs Wellcome Fund grant. S.M.G. is supported in part by NIH training grants T32-AI007419 and T32-AI007001. B.D. is a Leukemia & Lymphoma Society Scholar and Burroughs Wellcome Fund Investigator in Infectious Disease.
Addendum to:
References
- Engels EA, Pfeiffer RM, Goedert JJ, Virgo P, McNeel TS, Scoppa SM, et al. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS 2006; 20:1645 - 1654
- Schroder K, Tschopp J. The inflammasomes. Cell 2010; 140:821 - 832
- Davis BK, Wen H, Ting JP. The inflammasome NLRs in immunity, inflammation and associated diseases. Annu Rev Immunol 2011; 29:707 - 735
- Kanneganti TD. Central roles of NLRs and inflammasomes in viral infection. Nat Rev Immunol 2010; 10:688 - 698
- Randolph-Habecker J, Iwata M, Geballe AP, Jarrahian S, Torok-Storb B. Interleukin-1-mediated inhibition of cytomegalovirus replication is due to increased IFN beta production. J Interferon Cytokine Res 2002; 22:765 - 772
- Brennan MA, Cookson BT. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol Microbiol 2000; 38:31 - 40
- Fink SL, Cookson BT. Apoptosis, pyroptosis and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 2005; 73:1907 - 1916
- Johnston JB, Barrett JW, Nazarian SH, Goodwin M, Ricciuto D, Wang G, et al. A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 2005; 23:587 - 598
- Ray CA, Black RA, Kronheim SR, Greenstreet TA, Sleath PR, Salvesen GS, et al. Viral inhibition of inflammation: Cowpox virus encodes an inhibitor of the interleukin-1β converting enzyme. Cell 1992; 69:597 - 604
- Stasakova J, Ferko B, Kittel C, Sereinig S, Romanova J, Katinger H, et al. Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1{beta} and 18. J Gen Virol 2005; 86:185 - 195
- Gregory SM, Davis BK, West JA, Taxman DJ, Matsuzawa S, Reed JC, et al. Discovery of a viral NLR homolog that inhibits the inflammasome. Science 2011; 331:330 - 334
- Tanabe T, Chamaillard M, Ogura Y, Zhu L, Qiu S, Masumoto J, et al. Regulatory regions and critical residues of NOD2 involved in muramyl dipeptide recognition. EMBO J 2004; 23:1587 - 1597
- González CM, Wang L, Damania B. Kaposi's sarcoma-associated herpesvirus encodes a viral deubiquitinase. J Virol 2009; 83:10224 - 10233
- Sathish N, Zhu FX, Yuan Y. Kaposi's sarcoma-associated herpesvirus ORF45 interacts with kinesin-2 transporting viral capsid-tegument complexes along microtubules. PLoS Pathog 2009; 5:1000332