Abstract
Specialization into reproductive and non-reproductive castes is one of the defining traits of eusocial insects. Knowledge of the proximal causes of caste differentiation is therefore central to achieving an understanding of the evolution of eusociality. Castes are an example of a polyphenism, multiple, discrete phenotypes arising from a single genotype in response to differing environmental conditions. Here we focus on recent work in the social wasps to provide insight into how environmental conditions may trigger the development of caste across a range from independent- to swarm-founding social species. The amount of food larvae receive has long been recognized as a key input factor in the determination of caste, but that alone is insufficient to account for the range of combinations of size, development time, and caste among the female offspring of Polistes, an independent-founding wasp. Recent experimental work on P. fuscatus has shown that vibrations that are associated with the feeding of larvae are another essential environmental input in the determination of caste. We present a model of how vibrational signaling in the context of feeding larvae could interact with nutritional input to account for the developmental patterns seen in these wasps. Mapping the distribution of vibrational signaling onto a phylogeny of the social wasps suggests that this trait characterized the common ancestor of the subfamilies Vespinae + Polistinae, diversified in the independent-founding species, then was superseded by caste-determining mechanisms in the swarm-founding and vespine species that function more effectively in larger colonies.
The most fundamental specialization of the eusocial insects is the division of colony members into two castes, workers (functionally sterile individuals) and reproductives.Citation1 Understanding the proximal mechanisms leading to differentiation among individuals is therefore central to an understanding the evolution of eusociality itself.Citation2 Because the wasp family Vespidae includes species representing a range of degrees of sociality from solitary to swarm-founding, the wasps can provide clues about what the early evolutionary steps toward caste-determining mechanisms might have been.Citation3–Citation6
The degree of differentiation between the queen (reproductive) and worker castes in the wasps ranges from none in the subfamily Stenogastrinae to major differences in size and morphology in the Vespinae (hornets and yellowjackets). In species with morphologically distinct castes, it is clear that developmental differentiation between gynes (future queens) and workers must begin in the larval stages. In many members of the large subfamily Polistinae, including the independent-founding genera Polistes and Ropalidia, the castes are morphologically identical, but manifest physiological differences that also must originate in the larval stages.Citation7 This is supported by recent evidence that differences in the mRNA expression of several genes and in levels of a hexameric storage protein are already evident by the fifth (last) instar of larval development in Polistes metricus.Citation5,Citation8 There is no evidence for a genetic basis for caste differences in wasps. Instead, gyne and worker are examples of polyphenism, the epigenetic production within a single genotype of discrete forms of morphology, physiology or behavior in response to environmental differences.Citation9 Here we review recent advances in our understanding of the nature of the environmental conditions triggering caste differentiation in the social wasps and propose a new model.
In the highly dimorphic hornets and yellowjackets, it is thought that food amount coupled with pheromones or oral secretions fed to the larvae by the workers combine to trigger the divergence of paths.Citation10 For the non-dimorphic polistines such as Polistes, Ropalidia and others, the long-standing view is that differences in the quantity of nourishment received during the larval stage act as a “nutritional switch” to bias development toward one caste or the other.Citation7,Citation8,Citation11–Citation14 Female larvae fed ample food develop into gynes, whereas the less well-fed become workers.Citation4 Food-supplementation studies have shown that colonies given nutrients ad libitum produce higher frequencies of female offspring with gyne-like traits, including larger size, more fat body, greater cold tolerance and higher diapause potential, than control colonies.Citation15–Citation19 Conversely, under-feeding produces smaller offspring.Citation19 Workers, which are reared by the queen early in the colony cycle, are said to be the result of poor larval nutrition due to a low worker-to-larva (W/L) ratio at that time. The gynes, produced later, are the result of improved nutrition due to an increasing W/L ratio.Citation5,Citation13,Citation20,Citation21 Despite these pre-adult biasing effects, adults retain some behavioral and physiological plasticity to respond to the social context they find themselves in.Citation7
Limits of the Nutritional-Switch Hypothesis
A mechanism based strictly on the amount of food received by larvae appears to be too simplistic, even for Polistes. This single-dimensional variable is insufficient to account for the multi-variate patterns of development seen in this and other independent-founding polistines.Citation22 First, the rate of larval development, in insects generally positively correlated with resource availability,Citation9 does not correlate well with size or caste. In Polistes, Mischocyttarus and Ropalidia, larval development times are shortest for the first few (worker) offspring, rise rapidly to a maximum for later-emerging workers, then gradually decline to intermediate durations for the remainder of the colony cycle, when gynes are produced.Citation23–Citation27 Second, the first-produced workers not only develop more rapidly than any subsequently produced females, but paradoxically they are also the smallest.Citation24,Citation26 MeadCitation16 and KudoCitation28 concluded that the rapid growth of the first larvae in Polistes is due to more intensive feeding, but this is contradicted by studies providing supplemental feeding of larvae, which leads to both more rapid larval development and growth to larger, not smaller, adult size.Citation22 Furthermore, the larger females produced later in the colony cycle include workers that do not differ measurably in size from gynes.Citation21,Citation24 In other words, size, development rate and caste vary semi-independently of one another to produce more combinations than can be explained by the single input variable of food quantity.
Possible Role for Vibrational Signals
It has recently been suggested that vibrational signaling provides a second source of input that may modulate the effect of nutrition quantity.Citation22 Vibrational signals are especially conspicuous in the genera Polistes, Mischocyttarus, Ropalidia and Belonogaster. Females produce these signals either by rapidly shaking the body while standing on the nest, or by striking some part of the body against the nest. The movements are typically vigorous enough to produce audible sound and to cause the whole nest to shake or vibrate.Citation29,Citation30 Some categories of these signals are performed primarily by the dominant females while feeding the larvae. The best-known example is antennal drumming in Polistes, which is performed at the highest frequencies early in the colony cycle, when workers are being reared, and at much reduced rates later, when gynes are being reared.Citation31,Citation32 Recent experimental evidence for Polistes fuscatus has shown that larvae in nests subjected to simulated antennal drumming (pulses at 17 Hz) develop into adults with significantly less fat body than larvae given random-frequency vibration of the same intensity.Citation33 Low body fat is a worker-like trait, whereas high body fat is a characteristic of gynes.Citation34,Citation35 That vibrational signals can have this effect is not surprising. Mechanical stressors are known to have dramatic biochemical and developmental effects on insects and other animals.Citation22
We suggest the following scenario as one possible way nutritional and mechanical inputs could interact to lead to the observed patterns of caste, size and larval development time (). Early in the colony cycle, the first few queen-reared larvae are fed high amounts throughout their larval period,Citation16,Citation28 leading to their rapid development. The high frequency of feeding-coupled vibrations they are subjected to as larvaeCitation31,Citation32 inhibits the synthesis of fat storesCitation33 and other factors such as storage proteins that are essential for the capacity to undergo diapause,Citation5,Citation36 thus biasing their development toward a worker phenotype. Body size, which is loosely correlated with levels of fat and storage proteins, is reduced in these first emerging offspring.Citation34,Citation35 The next few cohorts of queen-reared offspring also emerge as workersCitation24 due to the continuing high levels of vibrations.Citation32 These workers have larger body sizesCitation24 because of the higher nutritional input they receive amidst seasonally improving food availabilityCitation21 and possibly to their greater total food intake due to their longer development time. Their longer development time as larvae may be due to low nutritional inputs received as 1st- and 2nd-instar larvae, when older larvae in the earlier cohorts were being preferentially fed. Later in the colony cycle, the worker-reared larvae experience high nutritional input due to the improved worker-to-larva ratio.Citation21 These larvae experience low levels of vibrations compared to the earlier queen-reared larvae.Citation31,Citation32 The combination of high nutritional input and low vibrational input causes these worker-reared offspring to develop rapidly, have larger body sizes and higher fat stores and storage protein levels, i.e., a gyne phenotype. Workers continue to emerge, albeit in decreasing numbers,Citation21 possibly due to uneven distribution of food along with individual differences in exposure to and sensitivity to vibrational stimuli.
Taxonomic Distribution of Vibrational Signaling
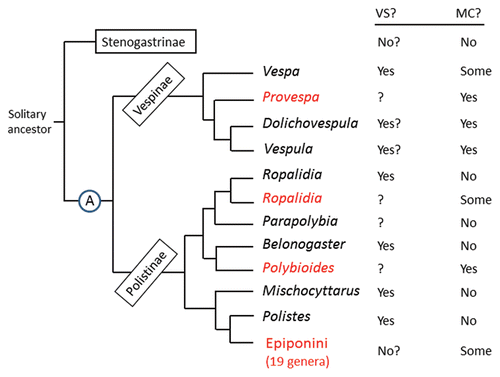
Mapping the taxonomic distribution of vibrational behavior in the context of feeding the larvae onto a phylogeny of the social Vespidae highlights its co-occurrence with independent-founding genera (). These genera are characterized by small colony sizes (typically well under 100 individuals) and absence of morphologically distinct castes.Citation37 Whether vibrational signals in these other genera play a caste-biasing role similar to that reported in Polistes remains to be investigated, but the principle of parsimony suggests that they do. Wasps in the less-derived subfamily Stenogastrinae form even smaller societies, lack reproductive and worker castes,Citation7 and also lack any evidence of vibrational signaling directed at the larvae.
The Vespinae present a more diverse picture (). Most form large colonies and show pronounced queen-worker dimorphism. The clearest account of vibrational signaling directed at the larvae is for Vespa tropica. When a founding queen returns to the nest with food for the larvae, she engages in prolonged tapping of the hind legs on the nest.Citation41 This species is unusual in that colonies are among the smallest in the subfamily, with 5–15 workers at maturity, and gyne and worker are not morphologically different, although as in Polistes the gynes have much more fat body than workers,Citation41 indicating caste differentiation originating in the immature stages. The more derived genera Vespula and Dolichovespula () are the most dimorphic of the social wasps, with gynes up to 40% larger and morphologically and physiologically distinct from workers.Citation37 Colonies are large and gynes are reared in specially constructed large cells, opening the possibility that workers could be cued to feed different amounts or qualities of food by the size of the cell a larva is in. Thus, a strictly nutritional switch could be acting, although pheromones are also suspected to play a role.Citation10 Interestingly, vibrational signals have been reported for a number of species. Workers of Vespula consobrina occasionally drum their abdomens (gasters) vigorously on the nest during rounds of brood-cell inspection.Citation42 This species also forms small colonies, with worker numbers of less than 100. Gaster drumming by founding queens or founding queens and workers has also been reported for Dolichovespula spp.Citation41,Citation43 and in young colonies of Vespula spp., although it is not clear whether it is associated with feeding the larvae.Citation44
The four taxa that initiate colonies by means of swarms of queens and workers () are also diverse, ranging from species with small colony size and lacking any queen-worker dimorphism or size differenceCitation45 to those with very large colonies and queens more than 20% larger than workers in some dimensions. Vibrational signaling directed at larvae has not been reported for any of the several hundred species in these groups, although most have not been studied at all. Queen-worker dimorphism in these species appears not to be derived from size-based allometry.Citation37 In Apoica, for example, queens and workers overlap completely in body size, yet differ in body proportions.Citation37 This means that the caste-differentiating mechanism cannot be triggered by a simple nutritional switch based on amount of food the larvae receive. Again, pheromones or oral secretions may be involved.
Evolutionary History of Mechanisms of Caste Differentiation
These patterns of occurrence begin to illuminate the evolution of environmental triggers of caste-determining developmental pathways. Vibrational signals appear to be a behaviorally applied mode of caste biasing that may be effective only in small colonies. They may have originated as unspecialized forms of mechanical stress, perhaps applied directly to the larvae. The more specialized signals characterizing extant species may be ritualized forms of this early behavior. One scenario is that vibrational caste-biasing characterized the common ancestor of the clade Vespinae + Polistinae (), diversified in form and function among the independent founders, then was superseded in the more derived taxa by other mechanisms, such as pheromones or differences in food quality. These may well be more effective mechanisms of caste biasing, able to affect hundreds or thousands of larvae, rather than a few tens. These mechanisms, which have evolved independently in several social vespid lineages, may have been instrumental in enabling the evolution of the larger colony sizes seen in the more derived vespines and in the several clades of swarm founders ().
Abbreviations
| W/L | = | worker-to-larva ratio |
| JH | = | juvenile hormone |
| IIS | = | insulin/insulin-like signaling pathways |
Figures and Tables
Figure 1 Model for the interaction of vibrational signaling and nutrition in caste biasing in Polistes. (A) Hypothesized changes in the temporal pattern of vibration and levels of nutrition received per larva per hour across the colony cycle.Citation32 (B) Correlated changes in the development time, body size and caste of emerging adults across the colony cycle.Citation21,Citation24 See text for explanation.
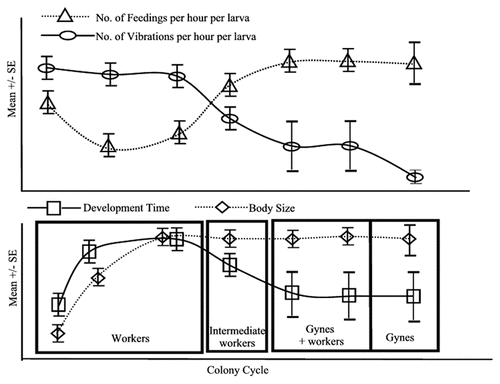
Figure 2 Cladogram of the eusocial groups of vespid wasps. VS?, occurrence or not of vibrational signals performed by adults in association with feeding the larvae. MC?, occurrence or not of morphological differences between queen and worker. Taxa shown in black are independent-founding wasps; those shown in red are swarm founders. Cladogram based on several authors.Citation38–Citation40
Acknowledgments
We are grateful to B.J. Taylor, T. Schueller and A. Toth for critically reading the manuscript. Research supported by the College of Agricultural and Life Sciences, University of Wisconsin-Madison.
References
- Oster GF, Wilson EO. Caste and Ecology in the Social Insects 1978; Princeton, NJ Princeton University Press
- Smith CR, Toth AL, Suarez AV, Robinson GE. Genetic and genomic analyses of the division of labour in insect societies. Nature Revs Gen 2008; 9:735 - 748
- West-Eberhard MJ. Alexander RD, Tinkle DW. Intragroup selection and the evolution of insect societies. Natural Selection and Social Behavior: Recent Research and New Theories 1981; Chiron, New York 3 - 17
- Hunt JH, Amdam GV. Bivoltinism as an antecedent to eusociality in the paper wasp genus Polistes. Science 2005; 308:264 - 267
- Hunt JH, Kensinger BJ, Kossuth JA, Henshaw MT, Norberg K, Wolschin F, et al. A diapause pathway underlies the gyne phenotype in Polistes wasps, revealing an evolutionary route to caste-containing insect societies. Proc Natl Acad Sci USA 2007; 104:14020 - 14025
- Toth AL, Varala K, Newman TC, Miguez FE, Hutchison SK, Willoughby DA, et al. Wasp gene expression supports an evolutionary link between maternal behavior and eusociality. Science 2007; 318:441 - 444
- O'Donnell S. Reproductive caste determination in eusocial wasps (Hymenoptera: Vespidae). Ann Rev Entomol 1998; 43:323 - 346
- Hunt JH, Wolschin F, Henshaw MT, Newman TC, Toth AL, Amdam GV. Differential gene expression and protein abundance evince ontogenetic bias toward castes in a primitively eusocial wasp. PLoS One 2010; 5:10674
- Nijhout HF. The control of body size in insects. Dev Biol 2003; 261:1 - 9
- Greene A. Ross KG, Matthews RW. Dolichovespula and Vespula. The Social Biology of Wasps 1999; Ithaca, New York Cornell University Press 263 - 305
- Wheeler DE. Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications. Am Nat 1986; 128:13 - 34
- Wheeler DE. Kikuchi T, Azuma N, Higashi S. One hundred years of caste determination in Hymenoptera. Genes, Behaviors and Evolution of Social Insects 2003; Sapporo, Japan Hokkaido University Press 35 - 53
- Gadagkar R, Bhagavan S, Chandrshekara K, Vinutha C. The role of larval nutrition in pre-imaginal biasing of caste in the primitively eusocial wasp Ropalidia marginata (Hymenoptera: Vespidae). Ecol Entomol 1991; 16:435 - 440
- Hunt JH. Hunt JH, Nalepa CA. Nourishment and social evolution in wasps sensu lato. Nourishment and Evolution in Insect Societies 1994; Boulder CO Westview Press 211 - 244
- Rossi AM, Hunt JH. Honey supplementation and its developmental consequences evidence for food limitation in a paper wasp Polistes metricus. Ecol Entomol 1988; 13:437 - 442
- Mead F, Habersetzer C, Gabouriaut D, Gervet J. Dynamics of colony development in the paper wasp Polistes dominulus Christ (Hymenoptera: Vespidae): The influence of prey availability. J Ethol 1994; 12:43 - 51
- Miyano S. Number, larval durations and body weights of queen-reared workers of a Japanese paper wasp, Polistes chinensis antennalis (Hymenoptera: Vespidae). Nat Hist Res 1990; 1:93 - 97
- Hunt JH, Dove MA. Nourishment affects colony demographics in the paper wasp Polistes metricus. Ecol Entomol 2002; 27:467 - 474
- Karsai I, Hunt JH. Food quantity affects traits of offspring in the paper wasp Polistes metricus (Hymenoptera: Vespidae). Env Entomol 2002; 31:99 - 106
- Reeve HK. Ross KG, Matthews RW. Polistes. The social biology of wasps 1991; Ithaca, New York Cornell University Press 99 - 148
- West-Eberhard MJ. The social biology of polistine wasps. Misc Pubs, Mus Zool, U Michigan 1969; 140:1 - 101
- Jeanne RL. Gadau J, Fewell J. Vibrational signals in social wasps: A role in caste determination?. Organization of Insect Societies 2009; Cambridge, MA Harvard University Press 241 - 263
- Jeanne RL. Social biology of the Neotropical wasp Mischocyttarus drewseni. Bull Mus Comp Zool Harvard Univ 1972; 144:63 - 150
- Klahn JE. Alternative reproductive tactics of single foundresses of a social wasp, Polistes fuscatus 1981; Iowa City, IA U Iowa Ph.D., diss.
- Kojima J. Growth and survivorship of preemergence colonies of Ropalidia fasciata in relation to foundress group size in the subtropics (Hymenoptera: Vespidae). Ins Soc 1989; 36:197 - 218
- Miyano S. Number of offspring and seasonal changes of their body weight in a paper wasp, Polistes chinensis antennalis Perez (Hymenoptera: Vespidae), with reference to male production by workers. Res Pop Ecol 1983; 25:198 - 209
- Strassmann JE, Orgren MCF. Nest architecture and brood development times in the paper wasp, Polistes exclamans (Hymenoptera: Vespidae). Psyche 1983; 90:237 - 248
- Kudo K. Growth rate and body weight of foundress-reared offspring in a paper wasp, Polistes chinensis (Hymenoptera: Vespidae): No influence of food quantity on the first offspring. Ins Soc 2003; 50:77 - 81
- Keeping MG. Social organization and division of labour in colonies of the polistine wasp, Belonogaster petiolata. Behav Ecol Sociobiol 1992; 31:211 - 224
- Pratte M, Jeanne RL. Antennal drumming behavior in Polistes wasps (Hymenoptera: Vespidae). Zeitschr Tierpsychol 1984; 66:177 - 188
- Brillet C, Tian-Chansky SS, Le Conte Y. Abdominal waggings and variation of their rate of occurrence in the social wasp, Polistes dominulus Christ I. Quantitative analysis. J Ins Behav 1999; 12:665 - 686
- Suryanarayanan S, Hantschel A, Torres CG, Jeanne RL. Changes in the temporal pattern of antennal drumming behavior across the Polistes fuscatus colony cycle (Hymenoptera, Vespidae). Ins Soc 2011; 58:97 - 106
- Suryanarayanan S, Hermanson JC, Jeanne RL. A mechanical signal biases caste development in a social wasp. Curr Biol 2011; 21:231 - 235
- Eickwort K. Separation of the castes of Polistes exclamans and notes on its biology (Hym.: Vespidae). Ins Soc 1969; 16:67 - 72
- Toth AL, Bilof KBJ, Henshaw MT, Hunt JH, Robinson GE. Lipid stores, ovary development and brain gene expression in Polistes females. Ins Soc 2009; 56:77 - 84
- Hunt JH, Buck NA, Wheeler DE. Storage proteins in vespid wasps: Characterization, developmental pattern and occurrence in adults. J Insect Physiol 2003; 49:785 - 794
- Jeanne RL. Kikuchi T, Azuma N, Higashi S. Social complexity in the Hymenoptera, with special attention to the wasps. Genes, Behaviors and Evolution of Social Insects 2003; Sapporo, Japan Hokkaido University Press 81 - 130
- Wenzel JW, Carpenter JM. Eggleton P, Vane-wright RI. Comparing methods: adaptive traits and tests of adaptation. Phylogenetics and Ecology 1994; London Academic Press 79 - 101
- Arevalo E, Zhu Y, Carpenter JM, Strassmann JE. The phylogeny of the social wasp subfamily Polistinae: evidence from microsatellite flanking sequences, mitochondrial COI sequence and morphological characters. BMC Evol Biol 2004; 4:8
- Pickett KM, Carpenter JM. Simultaneous analysis and the origin of eusociality in the Vespidae (Insecta: Hymenoptera). Arth Syst Phylog 2010; 68:3 - 33
- Matsuura M, Yamane S. Biology of the Vespine Wasps 1990; New York Springer-Verlag
- Akre RD, Reed HC, Landolt PJ. Nesting biology and behavior of the blackjacket, Vespula consobrina (Hymenoptera: Vespidae). J Kans Entomol Soc 1982; 55:373 - 405
- Akre RD, Garnett WB, MacDonald JF, Greene A, Landolt PJ. Behavior and colony development of Vespula pensylvanica and V. atropilosa (Hymenoptera: Vespidae). J Kans Entomol Soc 1976; 49:63 - 84
- Ross KG. Gastral vibrations in laboratory Vespula vulgaris and V. maculifrons colonies (Hymenoptera: Vespidae). Fla Entomol 1982; 65:187 - 188
- Noll F, Wenzel JW, Zucchi R. Evolution of caste in Neotropical swarm-founding wasps (Hymenoptera: Vespidae; Epiponini). Amer Mus Novit 2004; 3467:1 - 24