Abstract
Controlled management of protein levels and quality is essential for normal cellular function. Specific molecular chaperones and foldases monitor the levels and assist correct folding of proteins. The ubiquitin-proteasome system recognizes and degrades misfolded proteins that can otherwise be harmful to cells. However, when misfolded or aggregated proteins excessively accumulate, they may be sequestered to the microtubule-organizing center to form aggresomes. These may then be removed from cells by autophagocytosis. Abnormal protein accumulation and aggregation is a common hallmark of many neurodegenerative diseases. In a recent study, we provide evidence that specific transcript variants (TVs) of ubiquilin-1, which are genetically and functionally associated to Alzheimer’s disease (AD), regulate proteasomal and aggresomal targeting of presenilin-1 (PS1), a key player in AD pathogenesis. Our study together with current data provide interesting implications for ubiquilin-1 and its TVs in the pathogenesis of AD and other neurodegenerative diseases involving abnormal protein aggregation.
Abnormal accumulation and aggregation of proteins is a common hallmark of neurodegenerative diseases. Intraneuronal neurofibrillary tangles (NFT) and excessive accumulation of β-amyloid (Aβ) peptides as amyloid plaques in the brain are central pathological events in Alzheimer's disease (AD).Citation1 The pathogenesis of Parkinson's (PD) and Huntington's diseases (HD) involves formation of intracellular protein inclusions termed Lewy bodies and intranuclear inclusions of huntingtin protein containing expanded poly-glutamine (polyQ) repeat, respectively.Citation2,Citation3 The ubiquitin-proteasome system (UPS) recognizes and discards excessively accumulated or misfolded proteins that are potentially harmful to the cells.Citation4 These proteins are tagged with Lys48-linked poly-ubiquitin chains. The poly-ubiquitinated proteins are recognized by proteasome-associated ubiquitin receptors by their ubiquitin-interacting motif (UIM) and degraded.Citation5 However, under conditions of excessive accumulation of misfolded or aggregated proteins, the degradation capacity of the UPS may become compromised. Another pathway to dispose of these proteins is aggresome formation. Aggresomes are cytoplasmic inclusion bodies that are formed by active sequestration of protein aggregates along the microtubules to the microtubule-organizing center (MTOC) next to the nucleus.Citation6 Also, characteristic of aggresomes is the presence of polyubiquitinated proteins and components of the proteasome within in the aggresomal core. This is surrounded by a vimentin envelope. It is suggested that aggresome formation is a cytoprotective response to make aggregated proteins harmless and prevent their abnormal and unspecific protein interactions.Citation6,Citation7
Several proteins function as polyubiquitin shuttles that target polyubiquitinated proteins to proteasomal degradation. These proteins contain a ubiquitin-like domain (UBL) at one end and a ubiquitin-associated domain (UBA) in the other. The UBL domain mediates interaction with the proteasome UIM domain and the UBA domain binds polyubiquitinated proteins. One of these polyubiquitin shuttle proteins is ubiquilin-1 (Dsk2/hPLIC).Citation8
Ubiquilin-1 is a cytoplasmic protein implicated in the targeting of specific proteins, such as AD-associated presenilin-1 (PS1), to the proteasome for degradation.Citation9 PS1 and PS2 proteins play an elementary role in the pathogenesis of AD. PSs are catalytic components in the γ-secretase enzyme complex that generates Aβ.Citation10 Furthermore, missense mutations in PSEN1 and PSEN2 genes cause early-onset form of AD and lead to augmented Aβ production.Citation11,Citation12 Ubiquilin-1 has been shown to increase accumulation of PS1 and PS2 and decrease ubiquitination and degradation of PS2.Citation13,Citation14 Under proteasomal inhibition, ubiquilin-1 and PS2 were found to co-localize in aggresomes.Citation14 Mounting evidence suggests that ubiquilin-1 is also involved in the targeting of other neurodegenerative disease-associated proteins to aggresomes or autophagosomes.Citation15,Citation16 Ubiquilin-1 is present in the NFTs, Lewy bodies and intranuclear inclusions containing polyQ protein.Citation13 Together, these data imply that ubiquilin-1 plays a role in different neurodegenerative diseases involving abnormal protein accumulation.Citation13,Citation17
Ubiquilin-1 gene (UBQLN1) undergoes alternative splicing to generate four transcript variants (TV). TV1, containing 11 exons, is the full-length form of ubiquilin-1. TV2 lacks exon 8. TV3 lacks exons 2, 3 and 4 and thus the majority of the UBL domain. The smallest isoform, TV4, contains the first 3 exons but thereafter has a unique short C-terminus and thus lacks the UBA domain (). All the four TVs are expressed in human brain.Citation18 Our previous genetic studies showed that a specific UBQLN1 allelic variant, UBQ8i, is associated with increased risk for AD. The presence of this risk allele led to an increased ratio of TV2 to TV1 in AD brain.Citation19 Replication studies have subsequently revealed both positive and negative results related to genetic association of UBQLN1 with AD (see www.alzgene.org).Citation20 We and others have also demonstrated that ubiquilin-1 regulates intracellular trafficking of amyloid precursor protein (APP), the antecedent protein for Aβ and subsequently influences Aβ generation.Citation21,Citation22 Ubiquilin-1 responds to hypoxia-induced unfolded protein response (UPR), a stress condition when unfolded proteins accumulate and attenuates the induction a UPR-inducible transcription factor CHOP [C/EBP (CAAT/enhancer-binding protein) homologous protein].Citation23 In agreement with these data, we showed that under tunicamycin-induced endoplasmic reticulum (ER) stress, which leads to UPR, ubiquilin-1 TV1, TV2 and TV3 attenuated CHOP induction and increased cell survival.Citation18 Collectively, these data suggest that that ubiquilin-1 is involved in central pathogenic events in AD.
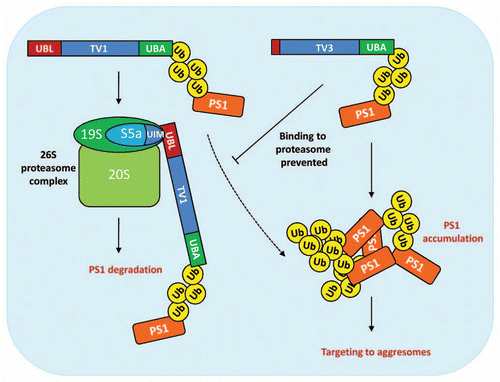
In our recent study, we investigated the effects of ubiquilin-1 full-length variant TV1 and TV3, which is devoid of most of the UBL domain, on the levels and subcellular localization of PS1 and activity of PS1-dependent γ-secretase.Citation24 We found that overexpression of TV3 with PS1 stabilized full-length PS1 levels and resulted in the accumulation of high-molecular-weight (HMW) forms of PS1 in human embryonic kidney (HEK293) cells. Furthermore, we observed that accumulated PS1 was targeted to aggresomes together with TV3. Although we did not detect HMW-PS1 formation in TV1-expressing cells, we found that PS1 and TV1 co-localized in the aggresomes. Moreover, formation of PS1-positive aggresomes was significantly increased in cells overexpressing TV1 and especially TV3 as compared to control cells. Similar observations were made in SH-SY5Y human neuroblastoma cells and embryonic mouse primary cortical neurons. Interestingly, overexpression of TV1 or TV3 in cells of glial lineage, such as H4 human neuroglioma cells or primary cortical astrocytes, did not result in the formation of aggresomes. These data suggest that different cells may have a differential propensity to utilize the aggresome pathway for discarding accumulated proteins. Altogether, our findings are in line with previous reports showing that ubiquilin-1 stabilizes PS1 levels and thus promotes HMW-PS1 accumulation.Citation13,Citation14 Our data further showed that accumulated PS1 was targeted to aggresomes by both TV1 and TV3. In contrast to previous studies, proteasomal inhibition was not required to stimulate aggresome formation in cells overexpressing TV1 or TV3 and PS1. Furthermore, we found no signs of a general UPS impairment in cells overexpressing TV1 or TV3 and PS1, demonstrating that PS1 accumulation in these cells was not caused by impeded proteasomal function. An interesting observation was that aggresomes were not formed in cells overexpressing TV1 or TV3 alone and that co-expression of PS1 was required. These data suggest that specific interaction of ubiquilin-1 TVs with an accumulated protein is required for the formation of ubiquilin-1-containing aggresomes.
Full-length PS1 levels are regulated by endoproteolysis and endogenous full-length PS1 is normally rarely detectable.Citation25 Our data suggest that the HMW-PS1 is formed as a result of full-length PS1 accumulation and that this leads to PS1 sequestration to aggresomes. Most likely, the endogenous low levels of full-length PS1 under normal conditions do not induce targeting of PS1 to aggresomes. This may explain why the cells overexpressing TV1 or TV3 alone, but not PS1, were devoid of aggresomes. Upon PS1 overexpression, however, the levels of full-length PS1 are drastically increased. These levels are further stabilized by TV1 or TV3. Accumulation of PS1 then likely activates the UPS and PS1 is targeted to the proteasome for degradation. In the case when the proteasome degradation capacity becomes overwhelmed, TV1 or TV3 directs PS1 to aggresomes. The fact that aggregated, poly-ubiquitinated proteins may be poor substrates to the proteasome may also contribute to the significantly increased aggresome formation in both TV1- and TV3-expressing cells. Furthermore, the aggresome pathway likely becomes predominant in TV3-overexpresing cells, as the interaction of TV3 with the proteasome is challenged due to the incomplete UBL domain (). Our electron microscope data indicated that TV1 and TV3 were also present in autophagosomes. Indeed, previous reports have shown that ubiquilin-1 co-localizes with LC3 (microtubule-associated protein 1 light chain 3), an autophagosomal marker protein and mediates autophagosomal delivery TDP-43 (43 kDa TAR DNA-binding domain protein), a protein associated with amyotrophic lateral sclerosis and fronto-temporal dementia.Citation26 Aggresomes have been suggested to be cleared from the cells by autophagocytosis.Citation6 Thus, our findings suggesting that TV1- and TV3-containing aggresomes are engulfed in autophagic vacuoles are in line with this idea.
Interestingly, in a polyQ model, deletion of ubiquilin-1 UBL domain prevented polyQ transport to aggresomes and the association of EPS15 (epidermal growth factor substrate 15), an endocytic protein, with dispersed aggregates. These data suggested that ubiquilin-1 UBL domain was required for the EPS15-mediated targeting of aggregated polyQ to the aggresomes.Citation15 On account of these results, one would assume that TV3 functioned in a dominant-negative manner to prevent aggresomal targeting of PS1. In contrast, we observed that TV3 enhanced PS1 targeting to the aggresomes as compared to TV1. The reason for these controversial data is currently unclear. TV3 still contains the N-terminal part of the UBL domain that is encoded by exon 1 (). It is possible that the remaining N-terminus of the UBL domain in TV3 is sufficient to mediate interaction with EPS15 and maintain efficient aggresomal targeting of PS1. On the other hand, it may be possible that the molecular players required for targeting of aggregated PS1 and polyQ proteins to the aggresomes are different and that EPS15 may not be necessary for PS1 aggresomal targeting.
Sequestration of PS1 to aggresomes in TV1- or TV3-overexpressing cells did not influence γ-secretase activity. Even though we observed decreased levels of secreted Aβ in HEK293 cells and increased Aβ levels in cultured primary cortical cells, we found no evidence for altered γ-secretase activity. This cell type-specific alteration of Aβ production in TV1- and TV3-expressing cells most likely results from differential effects of ubiquilin-1 on APP trafficking and maturation, as observed previously in different cells.Citation21,Citation22 Importantly, we noticed that in aggresome-containing cells, PS1 did not solely reside in aggresomes, but it was also strongly localized at or near the plasma membrane. It is therefore possible that this extra-aggresomal PS1 sustains normal γ-secretase activity in these cells. On the other hand, although TV1- and TV3-mediated targeting of PS1 to agresomes did not affect PS1-dependent γ-secretase activity, it is still plausible that other PS1-dependent cellular functions may be influenced in these cells. Further studies are required to understand and identify these possible alterations in PS1 function. Nevertheless, our findings together with previous data strongly point to an important role for ubiquilin-1 in fundamental molecular pathogenic events related to different neurodegenerative diseases. Furthermore, as different TVs of ubiquilin-1 appear to have distinct effects, regulation of UBQLN1 alternative splicing may affect ubiquilin-1 function also in neurodegeneration.
Figures and Tables
Figure 1 Ubiquilin-1 transcript variants (TV). TV1, the full-length form of ubiquilin-1 consists of 11 exons. The N-terminus of ubiquilin-1 protein harbors a UBL (ubiquitin-like) domain (shaded in red), which mediates interaction with the proteasome. The C-terminal UBA (ubiquitin-associated) domain binds poly-ubiquitinated proteins (shaded in green). TV2 lacks exon 8 (turquoise). TV3 lacks exons 2, 3 and 4 and thus the majority of the UBL domain. TV4 contains the first 3 exons. The frame shift leading to a 32-amino acid insertion after the exon 3/5 junction creates a unique short C-terminus (dark blue) and thus results in the lack of the UBA domain.
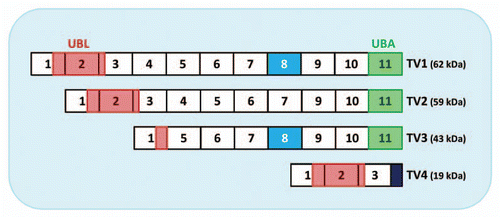
Figure 2 Schematic representation of the suggested function of ubiquilin-1 TV1 and TV3 in proteasomal and aggresomal targeting of PS1. TV1 binds poly-ubiquitinated PS1 via its UBA domain and shuttles PS1 to the 26S proteasome for degradation. UBL domain mediates the interaction between TV1 and the UIM (ubiquitin-interacting motif) domain of the proteasome 19S cap subunit S5a. Under excessive PS1 accumulation, TV1 may also target PS1 to the aggresomes (dashed arrow). TV3, which lacks majority of the UBL domain, is impeded in binding to the proteasome and predominantly targets accumulated PS1 to the aggresomes. Ub; ubiquitin.
Acknowledgments
This study was supported by EC FP6, MEST-CT-2005-019217 (to J.V.), the Health Research Council of the Academy of Finland (to A.H., H.S. and M.H.), EVO grant 5772708 of Kuopio University Hospital (to H.S.), the Nordic Centre of Excellence in Neurodegeneration (to H.S. and M.H.), Sigrid Juselius Foundation (to M.H.) and the strategic funding of the University of Eastern Finland.
Addendum to:
References
- Selkoe DJ. Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol 2004; 6:1054 1061
- Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson's disease and Parkinson's disease dementia. Acta Neuropathol 2010; 120:131 - 143
- Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol 2011; 10:83 - 98
- Mittal S, Ganesh S. Protein quality control mechanisms and neurodegenerative disorders: Checks, balances and deadlocks. Neurosci Res 2010; 68:159 - 166
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67:425 - 479
- Kopito RR. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol 2000; 10:524 - 530
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol 1998; 143:1883 - 1898
- Kleijnen MF, Shih AH, Zhou P, Kumar S, Soccio RE, Kedersha NL, et al. The hPLIC proteins may provide a link between the ubiquitination machinery and the proteasome. Mol Cell 2000; 6:409 - 419
- Ko HS, Uehara T, Tsuruma K, Nomura Y. Ubiquilin interacts with ubiquitylated proteins and proteasome through its ubiquitin-associated and ubiquitin-like domains. FEBS Lett 2004; 566:110 - 114
- De Strooper B, Annaert W. Novel research horizons for presenilins and gamma-secretases in cell biology and disease. Annu Rev Cell Dev Biol 2010; 26:235 - 260
- Sherrington R, Rogaev EI, Liang Y, Rogaeva EA, Levesque G, Ikeda M, et al. Cloning of a gene bearing missense mutations in early-on-set familial Alzheimer's disease. Nature 1995; 375:754 - 760
- Levy-Lahad E, Wasco W, Poorkaj P, Romano DM, Oshima J, Pettingell WH, et al. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science 1995; 269:973 - 977
- Mah AL, Perry G, Smith MA, Monteiro MJ. Identification of ubiquilin, a novel presenilin interactor that increases presenilin protein accumulation. J Cell Biol 2000; 151:847 - 862
- Massey LK, Mah AL, Ford DL, Miller J, Liang J, Doong H, et al. Overexpression of ubiquilin decreases ubiquitination and degradation of presenilin proteins. J Alzheimers Dis 2004; 6:79 - 92
- Heir R, Ablasou C, Dumontier E, Elliott M, Fagotto-Kaufmann C, Bedford FK. The UBL domain of PLIC1 regulates aggresome formation. EMBO Rep 2006; 7:1252 - 1258
- N'Diaye EN, Kajihara KK, Hsieh I, Morisaki H, Debnath J, Brown EJ. PLIC proteins or ubiquilins regulate autophagy-dependent cell survival during nutrient starvation. EMBO Rep 2009; 10:173 - 179
- Doi H, Mitsui K, Kurosawa M, Machida Y, Kuroiwa Y, Nukina N. Identification of ubiquitin-interacting proteins in purified polyglutamine aggregates. FEBS Lett 2004; 571:171 - 176
- Lu A, Hiltunen M, Romano DM, Soininen H, Hyman BT, Bertram L, et al. Effects of ubiquilin 1 on the unfolded protein response. J Mol Neurosci 2009; 38:19 - 30
- Bertram L, Hiltunen M, Parkinson M, Ingelsson M, Lange C, Ramasamy K, et al. Family-based association between Alzheimer's disease and variants in UBQLN1. N Engl J Med 2005; 352:884 - 894
- Bertram L, McQueen MB, Mullin K, Blacker D, Tanzi RE. Systematic meta-analyses of Alzheimer disease genetic association studies: the AlzGene database. Nat Genet 2007; 39:17 - 23
- Hiltunen M, Lu A, Thomas AV, Romano DM, Kim M, Jones PB, et al. Ubiquilin 1 modulates amyloid precursor protein trafficking and Abeta secretion. J Biol Chem 2006; 281:32240 - 32253
- Zhang C, Khandelwal PJ, Chakraborty R, Cuellar TL, Sarangi S, Patel SA, et al. An AICD-based functional screen to identify APP metabolism regulators. Mol Neurodegener 2007; 2:15
- Ko HS, Uehara T, Nomura Y. Role of ubiquilin associated with protein-disulfide isomerase in the endoplasmic reticulum in stress-induced apoptotic cell death. J Biol Chem 2002; 277:35386 - 35392
- Viswanathan J, Haapasalo A, Bottcher C, Miettinen R, Kurkinen KM, Lu A, et al. Alzheimer's Disease-Associated Ubiquilin-1 Regulates Presenilin-1 Accumulation and Aggresome Formation. Traffic 2011; 12:330 - 348
- Podlisny MB, Citron M, Amarante P, Sherrington R, Xia W, Zhang J, et al. Presenilin proteins undergo heterogeneous endoproteolysis between Thr291 and Ala299 and occur as stable N- and C-terminal fragments in normal and Alzheimer brain tissue. Neurobiol Dis 1997; 3:325 - 337
- Kim SH, Shi Y, Hanson KA, Williams LM, Sakasai R, Bowler MJ, et al. Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1. J Biol Chem 2009; 284:8083 - 8092