Abstract
The corneal epithelium generates a significant trans-epithelial potential (TEP) which aids in maintaining cornea water balance and transparency. Injury to the cornea causes a short circuit of the TEP at the wound. The TEP in the intact epithelium around the wound acts like a battery, powering significant ion flux and electric current at the wound. These circulating endogenous currents generate an electric field orientated towards the wound, with the wound the cathode. Many cell types, including human corneal epithelial cells and keratinocytes, migrate to the cathode at physiological electric field strengths. Indeed, the electric signal is a powerful stimulator of cell migration which appears to over-ride other cues such as chemotaxis and wound void. These wound fields also have a dynamic timecourse of change after wounding. It has been assumed that wound electric fields are produced by passive leakage of ions from damaged cells and tissue. Could these fields be actively maintained and regulated as an active wound response? What are the molecular, ionic and cellular mechanisms underlying the wound electric currents?
Electric fields and currents at human skin wounds were first measured many years ago.Citation1,Citation2 These electric signals are naturally-occurring and long-lasting, and are present in many, if not all, epithelia. The corneal epithelium has a significant endogenous trans-epithelial potential (TEP) of up to 45 mV, generated by active pumping of sodium and chloride, and maintained by tight junctions between the corneal epithelial cells. Damage to the cornea causes a short-circuit of the TEP at the wound resulting in large outward electric currents at the wound edge.Citation3 These currents are sustained by the TEP in the surrounding intact epithelium acting like a battery (). Thus, the cornea wound is surrounded by electric fields orientated towards the wound, with the wound the cathode (negative pole). Our laboratory and others have shown that many cell types, including human corneal epithelial cells and keratinocytes, migrate to the cathode in vitro at physiological field strengths.Citation4–Citation7 We have also shown that enhancing or inhibiting wound electric fields pharmacologically significantly increases or decreases cornea wound healing, respectively.Citation3
Active transport of sodium and chloride across the corneal epithelium is the basis of the TEP, and we have shown that substituting Na+ or Cl− in the bathing artificial tear solution significantly alters wound electric currents.Citation3,Citation8 We postulated that chloride-free eye drops may promote corneal wound healing in elderly or diabetic patients. The cornea wound electric current (by definition flow of positive charge) is generated by the combined flux (flow) of a number of charged species, both positively- and negatively-charged. The present study aimed to enhance and extend our knowledge of the ion species involved in carrying the cornea wound electric currents, and whether the flux is passive leakage or active transport. By defining the electrophysiological properties of the cornea we will be able to understand the mechanisms of generation of the endogenous wound electric fields and offer new approaches to manage wound healing and corneal injury.
We used ion-selective self-referencing microelectrodesCitation9,Citation10 to measure flux of a number of specific ions (Na+, Cl−, Ca2+, K+) at rat cornea wounds in vitro. The electrode contains an ionophore that makes it sensitive to a specific ion. Attached to a computer-controlled micropositioner, the electrode oscillates at low frequency (0.3 Hz) between two points 30 µm apart. If a flow of ions is present, the electrode detects a difference in ion concentration between its two positions. The actual ion flux can be calculated using the formula: J = Cu(dc/dx) where C is the ion concentration in the solution, u is the ion mobility, and dc is the concentration difference over distance dx. Ion flux data are presented in pmol/cm2/sec or nmol/cm2/sec. In drug-treatment experiments, eyes were kept in drug for 20 min prior to measurements. We also used molecular biology and immunohistochemistry to study expression and distribution of ion channels, and used a vibrating probe to measure wound electric current in the presence of ion channel blockers. The vibrating probe is a metallic electrode which vibrates at high frequency. If an electric current is present due to ion flux, the charge on the electrode fluctuates in proportion to the size of the current. The probe is connected to a lock-in amplifier that locks on to the probe's specific frequency signal. The probe is calibrated in a current density of 1.5 µA/cm2 at the start and end of experiments.Citation11
Cornea wounds showed an efflux (outward flow) of calcium ions which increased for the first 20 min after wounding, then plateaued at ∼5 pmol/cm2/sec. This steady increase after wounding, and the observation that fixing the tissue abolished the Ca2+ flux, suggests that Ca2+ flux may be regulated at the wound. Potassium efflux, on the other hand, peaked immediately after wounding then declined rapidly. High K+ in the artificial tear solution reversed the efflux to become an influx. These data suggest that K+ flux at the wound is passive leakage from damaged cells and tissue at the wound (K+ has a ten-fold higher intracellular concentration than any other ion). Surprisingly, we observed an influx of sodium ions at the wound which was maintained up to 40 min after wounding. However, treatment with drugs (aminophylline, ascorbic acid) which we have shown to increase cornea wound current and healing rate,Citation3 reversed this to give a N+ efflux. So the effect of these drugs to increase wound current is partly due to reversal and stimulation of Na+ efflux.
By far the largest ion flux we measured was chloride flux. The large Cl− influx increased steadily up to 60 min after wounding. Note that influx of a negative ion translates to an outward flow of positive electric current. Aminophylline significantly enhanced the Cl− influx (p < 0.03). Because of this large and increasing Cl− flux we measured cornea wound electric current with a vibrating probe in the presence of broad-spectrum chloride channel blocker DIDS (4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid disodium salt hydrate).Citation12 DIDS significantly reduced (halved) the wound current (p < 0.03) () indicating a large contribution of chloride flux to the overall cornea wound electric current. Overlaying the combined ion flux data (all ions) with the timecourse of wound electric currentCitation3 shows that cornea wound electric current increase after wounding is due to increasing ion flux (). By plotting the individual ion flux data (and the combined ion flux data) we can see that Cl− is the major ion contributing to the normal cornea wound electric current (). Enhancement of wound current by aminophylline is partly due to reversal of Na+ flux (inward to outward) but mainly by enhancement of Cl− influx.
We then investigated the distribution and expression of Cl− channel CLC2, because it is expressed abundantly and specifically in corneal epithelial tissue.Citation13 In unwounded corneas, CLC2 channels were seen predominantly in the apical layers of the corneal epithelium (). One hour after wounding fluorescence was increased throughout the entire thickness of the epithelium, showing redistribution and increased concentration of CLC2 channels close to the wound (). We also used quantitative PCR (qPCR) to detect CLC2 channel mRNA in human corneal epithelial cell monolayer before and 60 min after wounding. After scratch wounding, CLC2 mRNA expression was significantly increased (p < 0.05) (). Interestingly, the timecourse of upregulation correlated with the increase of chloride flux, which reached its maximum value one hour after wounding.
Because of the unexpected large contribution of inward chloride flux to the cornea wound electric current, we used the vibrating probe to investigate the effect of a number of other chloride channel blockers on the wound electric current. Niflumic acid, a CLC1 blocker, had no effect. Mefloquine and fluoxetine, inhibitors of volume-sensitive and calcium-activated Cl− channels, also had no effect. Surprisingly, NPPB, a non-specific blocker which is a potent inhibitor of epithelial Cl− conductanceCitation14 also had no effect on wound current. The Cl− channel blocker DIDS, that halved the wound current (see above and ), is a broad-spectrum, generic inhibitor which blocks a number of Cl− channels, including some members of the CLC family.Citation15 It is unlikely that CLC2 channels alone generate the large chloride flux. It will require further study to elucidate all the channels involved.
Our results challenge the conventional assumption that wound electric currents are merely ion leakage due to breakdown of the epithelial barrier, because: (1) flux of some ions (Ca2+, Cl−) increases steadily for up to 60 min after wounding (passive leakage would give an initial flux soon after wounding which then decreases). (2) Fixing tissue eliminates flux. (3) Pharmacological drugs which enhance ion pumping increase ion flux. (4) Timecourse of Cl− flux increase correlates with upregulation and re-distribution of CLC2 chloride channel. (5) Chloride channel blocker DIDS significantly reduced (halved) wound electric current.
In summary, wounding the cornea induced fluxes of Ca2+, K+, Na+ and Cl− with distinct time courses, which can be modulated by fixation or pharmacological treatment. Increased transport of Cl− is a rapid response to corneal injury and forms a significant portion of the wound electric current. Electric signaling at wounds appears to be an active signaling mechanism which may be exploited to induce and accelerate wound healing. Defining the ionic and molecular foundations of wound electric field generation offers potential novel approaches to modulate wound healing, e.g., to develop eyedrops targeting ion transport to aid in the challenging management of non-healing corneal ulcers. We have recently shown that wound currents can be pharmacologically enhanced in human corneas.Citation8 This opens the way to future clinical trials to test chloride-free or drug-containing eyedrops in human patients.
Figures and Tables
Figure 1 Which ions contribute to the wound electric current? The corneal epithelium transports Na+ and Cl− to generate and maintain a transepithelial potential difference (TEP). Injury breaks the epithelial barrier and collapses the potential at the wound (left). The positive potential in the surrounding intact epithlium drives ion current flow out of the wound (blue arrows) and forms laterally-orientated wound electric fields (red arrow) with the wound the cathode. These fields initiate and promote healing by stimulating cell migration into the wound.
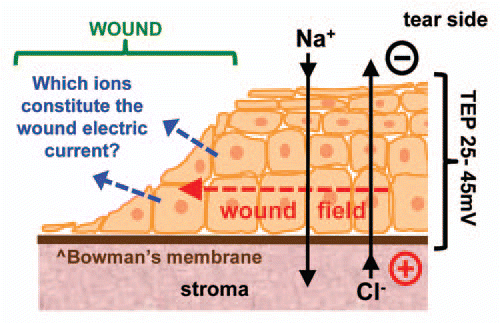
Figure 2 Relative contributions of ions to wound current. (A) Chloride channel blocker DIDS significantly reduces cornea wound electric current (*p < 0.03). (B) Overlaying wound electric current data (red) with compiled ion flux data (flux of all ions combined; blue) shows that electric current increase after wounding is due to an increasing ion flux (mainly chloride). Wound electric current data from Reid et al.Citation3 (C) Chloride is the major ion contributing to the normal (“Control”; blue) wound current. Enhancement of wound current by aminophylline (“Amin.”; red) is mostly due to stimulation of chloride flux, but also partly by reversal of sodium flux (inward to outward).
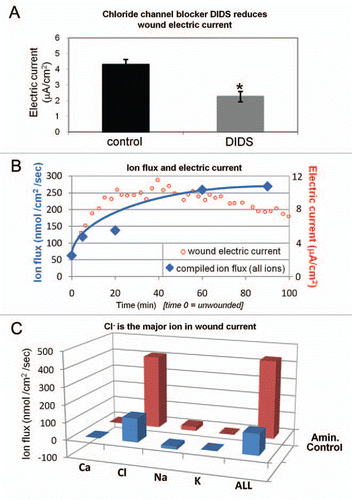
Figure 3 Distribution and expression of calcium-activated chloride channel-2 (CLC2). (A) In unwounded cornea, CLC2 channels were concentrated in the superficial epithelial cells (arrowhead). (B) One h after wounding, fluorescence was present throughout the entire thickness of the epithelium, showing re-distribution and increased concentration of CLC2 channels. Scale bars 50 µm. (C) In human corneal epithelial cell monolayer, scratch wounding induced increased expression of CLC2 channel mRN A (*p < 0.05).
Acknowledgments
This work was supported by the National Institutes of Health National Eye Institute grant 1R01EY019101 (to M.Z. and B.R.). The authors thank the Wellcome Trust for continuous support (068012). This work was also supported in part by Research to Prevent Blindness, Inc., an NSFC grant (30628026), and U.C. Davis Dermatology Department developmental fund. M.Z. is also supported by grants from the California Institute of Regenerative Medicine RB1-01417, NSF MCB-0951199.
Addendum to:
References
- Du Bois-Reymond E. Vorläufiger abriss einer untersuchung uber den sogenannten froschstrom und die electomotorischen fische. Ann Phy U Chem 1843; 58:1 - 30
- Du Bois-Reymond E. Untersuchungen uber thierische Elektricitat, Zweiter Band, Zweite Abtheilung (Erste Lieferung) 1860; Berlin Georg Reimer
- Reid B, Song B, McCaig CD, Zhao M. Wound healing in rat cornea: the role of electric currents. FASEB J 2005; 19:379 - 386
- Zhao M, Agius-Fernandez A, Forrester JV, McCaig CD. Directed migration of corneal epithelial sheets in physiological electric fields. Invest Ophthalmol Vis Sci 1996; 37:2548 - 2558
- Zhao M, McCaig CD, Agius-Fernandez A, Forrester JV, Araki-Sasaki K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr Eye Res 1997; 16:973 - 984
- Farboud B, Nuccitelli R, Schwab IR, Isseroff RR. DC electric fields induce rapid directional migration in cultured human corneal epithelial cells. Exp Eye Res 2000; 70:667 - 673
- Nishimura KY, Isseroff RR, Nuccitelli R. Human keratinocytes migrate to the negative pole in direct current electric fields comparible to those measured in mammalian wounds. J Cell Sci 1996; 109:199 - 207
- Reid B, Graue-Hernandez EO, Mannis MJ, Zhao M. Modulating endogenous electric currents in human corneal wounds—a novel approach of bioelectric stimulation without electrodes. Cornea 2011; 30:338 - 343
- Smith PJS. Non-invasive ion probes—tools for measuring transmembrane ion flux. Nature 1995; 378:645 - 646
- Smith PJS, Hammar K, Porterfield DM, Sanger RH, Trimarchi JR. Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. Microsc Res Technique 1999; 46:398 - 417
- Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nature Protoc 2007; 2:661 - 669
- Okada Y, Shimizu T, Maeno E, Tanabe S, Wang X, Takahashi N. Volume-sensitive chloride channels involved in apoptotic volume decrease and cell death. J Membr Biol 2006; 209:21 - 29
- Cao L, Zhang XD, Liu X, Chen TY, Zhao M. Chloride channels and transporters in human corneal epithelium. Exp Eye Res 2010; 90:771 - 779
- Kirkup AJ, Edwards G, Weston AH. Investigation of the effects of 5-nitro-2-(3-phenylpropylamino)-benzoic acid (NPPB) on membrane currents in rat portal vein. Br J Pharmacol 1996; 117:175 - 183
- Pusch M, Zifarelli G, Murgia AR, Picollo A, Babini E. Channel or transporter? The CLC saga continues. Exp Physiol 2006; 91:149 - 152