Abstract
GSH is mostly considered a non-enzymatic antioxidant that serves for modulating the redox status of protein thiols, detoxification, and direct scavenging activity of oxyradicals. Within the cells, GSH has also the role to buffer the flux of nitric oxide (NO), which in the nervous system is physiologically produced being an important neuromodulator and neurotransmitter. However, this role of GSH in modulating NO toxicity is often considered of secondary importance. Recently, we confuted such assumption as we demonstrated that GSH depletion triggers a severe NO imbalance, which is the primary cause of neuronal death. Here we report that even a slight and non-toxic decrease of GSH in brain mice causes protein nitration that is reversed by inhibiting NO production. This evidence indicates that NO imbalance and the associated nitrosative hallmarks observed in neurodegenerative diseases as well as in health ageing are likely the consequence of the progressive decline of GSH.
NO is a physiologically-produced molecule having a multifaceted and pleiotropic function. In the nervous system it plays a role in neurotransmitter release, neural development, regeneration, synaptic plasticity and regulation of gene expression. Due to its intrinsic reactive feature, NO could also exert pro-apoptotic and pro-oxidant function. NO and its derived reactive nitrogen species (RNS) oxidize and damage DNA, membranes and proteins, and modulate the expression of genes and the activity of proteins that manage apoptosis including central-signal responsive transcription factors such as p53, NFκB and AP-1.Citation1,Citation2 The NO synthesis and the site of its production must be tightly regulated to prevent such adverse effects. For instance, in neuronal cells the anchoring of neuronal NO synthase (nNOS) to plasma membrane allows the interaction with NMDA receptor and PSD-95/93 and the immediate coupling of NO synthesis to glutamate signaling and synaptic plasticity. Also enzymatic antioxidants may prevent NO toxicity among which superoxide dismutases (SODs) perhaps represent the most effective.Citation3 Indeed, by scavenging superoxide, SODs limit the formation of peroxynitrite that mostly derives from the encounter of superoxide with NO. Glutathione (GSH) is the major non-enzymatic antioxidant regulating intracellular redox environment and is principally considered to have a crucial role in protection against oxidative stress. GSH pool declines with ageing and this event may be either the consequence of its scavenging/detoxifying activity or inactivation of its synthesizing enzymes.Citation4,Citation5 Many neurodegenerative diseases and the physiological aging are both characterized by GSH decrease in the brain and the accumulation of oxidative and nitrosative damage. We recently demonstrated that neuronal cells are highly vulnerable to physiological flux of NO in the absence of GSH.Citation6 In essence we observed that chemical inhibition of GSH synthesis induces damage to DNA and proteins and cell death both in neuroblastoma and primary cortical neurons, which is completely prevented by inhibiting NO synthase activity. These results finely indicate that nullifying the toxic effects of NO represents the primary role of GSH at least in neuronal cells.
To more thoroughly support such idea, we moved at examining the effects of GSH depletion in C57/BL6 mice. To inhibit GSH and NO synthesis, L-Buthionine Sulfoximine (BSO) and/or NG-Nitro-L-Arginine Methyl-Ester (L-NAME) were added in drinking water at concentration of 20 mM and 4 mM respectively. After one month mice were sacrificed according to the Animal Research Guidelines of the European Communities Council Directive (86/609/EEC) and GSH content was measured in various organs and tissues by HPLC. As reported in , GSH significantly decreased in all the organs and tissues screened (liver, heart, brain, skeletal, muscle and lung) but at different extent. Consistent with the poor BSO transportation into the brain,Citation7 GSH in this district was reduced of about 15% with respect to control. Intriguingly, such slight decrease was sufficient to induce nitrative damage to proteins as detected by dot blot using an anti-3-nitrotyrosine (3-NT) antibody. In agreement with the results previously obtained in neuroblastoma and cortical neurons, the inhibition of NO production by L-NAME massively prevented protein nitration in brain (). Paradoxically, the occurrence of 3-NT accumulation was not detected in the other organs (data not shown) albeit they displayed a more pronounced depletion of GSH (). This discrepancy can be due to the intrinsic nature of brain metabolism that produces higher flux of oxyradicals and RNS.Citation8 However, the slight drop of GSH concentration in brain was not still able to activate apoptotic pathways. Indeed, apoptotic markers such as Bax increase, pro-caspase-3 and Bcl-2 decrease or AIF accumulation into mitochondria were not observed ( and C). These results strongly suggest that in the brain even a trivial GSH deficiency causes protein nitration and such damage can be considered a predictive marker of neuronal apoptosis. Therefore, it can be assumed that a slight and/or sporadic decrease of intracellular GSH content—physiologically occurring during the metabolic activity of the brain—can irreversibly affect protein integrity and represents one of the main early causes of neuronal loss in ageing and neurodegenerative diseases.
The importance of sulfur amino acid and especially cysteine that is the amino acid precursor of GSH, in human health has been largely addressed and for this reason a dietary intervention to prevent the natural GSH decline with elderly assumes pivotal importance.Citation9 However, additional knowledge of the nutritional regulation of GSH metabolism are still necessary and critical for the development of effective strategies to improve human health.
Figures and Tables
Figure 1 Glutathione depletion is associated with 3-nitrotyrosine increase in mice brain without accumulation of apoptotic markers. (A) Left part: 20 µg of proteins extracted from mice brains were spotted on nitrocellulose membrane and subjected to Dot blot analysis using a polyclonal 3-nitrotyrosine (3-NT) antibody. Right part: density of immunoreactive dots was normalized for Ponceau Red spots and reported as arbitrary units (a.u.) and as means ± SD. (*p < 0.01 versus control, **p < 0.01 versus BSO-treated; n = 3). (B) 20 µg of total protein extracts were loaded for detection of Bcl-2, Bax and pro-caspase-3 by western blot. Actin was used as loading control. (C) mitochondria were purified from mice brains homogenates by Percoll® gradient, lysed and 20 µg of proteins were loaded for detection of AIF by western blot. Cytochrome c was used as loading control.
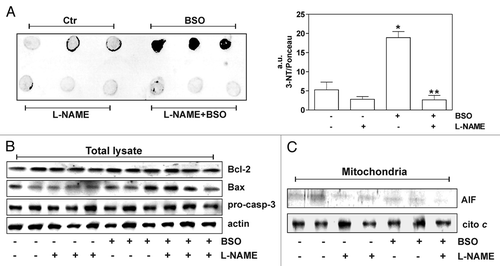
Table 1 GSH concentration in mice treated with BSO
Acknowledgments
This work was partially funded by grants from Ministero della Salute (#GR-2008-1138121) and MIUR.
Addendum to:
References
- Calabrese V, Cornelius C, Rizzarelli E, Owen JB, Dinkova-Kostova AT, Butterfield DA. Nitric oxide in cell survival: a janus molecule. Antioxid Redox Signal 2009; 11:2717 - 2739
- Kroncke KD. Nitrosative stress and transcription. Biol Chem 2003; 384:1365 - 1377
- Rotilio G, Aquilano K, Ciriolo MR. Interplay of Cu,Zn superoxide dismutase and nitric oxide synthase in neurodegenerative processes. IUBMB Life 2003; 55:629 - 634
- Ballatori N, Krance SM, Notenboom S, Shi S, Tieu K, Hammond CL. Glutathione dysregulation and the etiology and progression of human diseases. Biol Chem 2009; 390:191 - 214
- Sastre J, Martin JA, Gomez-Cabrera MC, et al. Age-associated oxidative damage leads to absence of gamma-cystathionase in over 50% of rat lenses: relevance in cataractogenesis. Free Radic Biol Med 2005; 38:575 - 582
- Aquilano K, Baldelli S, Cardaci S, Rotilio G, Ciriolo MR. Nitric oxide is the primary mediator of cytotoxicity induced by GSH depletion in neuronal cells. J Cell Sci 2011; 124:1043 - 1054
- Fekete I, Griffith OW, Schlageter KE, Bigner DD, Friedman HS, Groothuis DR. Rate of buthionine sulfoximine entry into brain and xenotransplanted human gliomas. Cancer Res 1990; 50:1251 - 1256
- Ikonomidou C, Kaindl AM. Neuronal death and oxidative stress in the developing brain. Antioxid Redox Signal 2011; 14:1535 - 1550
- Droge W, Kinscherf R, Hildebrandt W, Schmitt T. The deficit in low molecular weight thiols as a target for antiageing therapy. Curr Drug Targets 2006; 7:1505 - 1512