Abstract
Kinetochores must continuously associate with dynamic microtubule plus ends, as they oscillate along the mitotic spindle. The molecular basis for the kinetochore to track microtubule plus ends remains unresolved. In a recent study, we have shown an essential role of the formin mDia3 in stable kinetochore microtubule attachment and metaphase chromosome alignment. This function is attributable to EB1-binding by mDia3, for replacing endogenous mDia3 with an EB1-binding deficient mutant results in chromosome misalignment. EB1 specifically targets to attached, antipoleward kinetochores with polymerizing microtubules during chromosome oscillation. Therefore, we speculate that the mDai3-EB1-APC complex formation may relay EB1 microtubule plus end-tracking activity to the kinetochore.
Chromosome movement on the spindle during mitosis is powered and regulated by the kinetochore, a macromolecular protein complex that assembles on the centromere.Citation1 Sister kinetochores of a duplicated chromosome pair capture microtubules from the opposite spindle poles to the cell center. During metaphase chromosome oscillation, the leading and trailing kinetochores in a sister kinetochore pair have two distinct stably attached microtubule populations: the trailing kinetochore is attached to growing microtubules, whereas the leading one retains its attachment to shrinking microtubules. Numerous proteins and protein complexes have been implicated in connecting kinetochores with spindle microtubules.Citation1–Citation3 However, the most important missing piece is how the kinetochore tracks growing and shrinking microtubule plus ends.
A current model proposes a ternary KMN network (the KNL-1, Ndc80 and Mis12 complexes) to serve as a core microtubule binding apparatus at the kinetochore for stabilizing end-on microtubule attachment (reviewed in Joglekar et al.). The Ndc80 complex possesses a microtubule-binding activity with a weak affinity and is unable to recognize microtubule ends in in vitro assays.Citation4–Citation6 A recent study has presented an in vitro plus end-tracking system with purified yeast Dam1 and Ndc80 complexes.Citation7 The Dam1 complex, but not the Ndc80 complex, has an intrinsic microtubule plus end-tracking ability and is able to mediate continuous Ndc80 tip association with dynamic microtubules.Citation7 These results suggest that in yeast, the Dam1 complex can confer microtubule plus end-tracking activity to the Ndc80 complex, thus mediate the kinetochore tracking of microtubule plus ends. So far no Dam1 homolog has been identified in mammals.
EB1, a microtubule plus-end tip tracking protein, has been shown to associate with the trailing kinetochore (in a sister kinetochore pair) where there is net kinetochore microtubule growth (polymerization),Citation8 and is important for stable kinetochore-microtubule attachment.Citation9–Citation11 The interaction with growing microtubule plus ends is an intrinsic property of EB1,Citation12,Citation13 but the mechanism and the role for its stable association with the trailing sister kinetochore is not clear. In our recent study, we discovered that the interaction between EB1 and a kinetochore associated formin protein, mDia3, is essential for kinetochore microtubule attachment and metaphase chromosome alignment.Citation14 mDia formins have been shown in conjunction with EB1, and its binding partner APC, in the regulation of microtubule capture in different cell contexts.Citation15 Microtubule capture at bud sites in budding yeast is regulated by the formin Bni1, the yeast ortholog of mDia.Citation16,Citation17 The microtubule tip-binding protein Bim1 (the yeast ortholog of EB1) and Kar9 (speculated to represent a possible APC homolog) have been identified in this process.Citation18–Citation21 An evolutionarily conserved pathway for microtubule capture at the leading edge of migrating fibroblasts has been described, in which mDia formin functions as a scaffold protein for EB1 and APC.Citation22 Using an mDia3 mutant that cannot bind to EB1, we have shown that the interaction between EB1 and mDia3 is essential for metaphase chromosome alignment.Citation14
EB1 and APC only localize at microtubule-attached kinetochores.Citation23 In contrast, mDia3 is a novel kinetochore protein, which associates with kinetochores from prometaphase to metaphase.Citation14,Citation24 The kinetochore localization of mDia3 is microtubule independent as it localizes at unattached kinetochores upon nocodazole treatment (to disassemble microtubules).Citation14 Depletion of Aurora B using siRNA or inhibition of Aurora B kinase activity using a small molecule (ZM447439) does not affect mDia3 recruitment onto kinetochores, either (Zhang J and Mao Y, unpublished data). Furthermore, we observed co-immunoprecipitation between mDia3 and Hec1 (Cheng L and Mao Y, unpublished data), a component of the Ndc80 complex, from mitotic cell lysates, indicating a potential physical interaction between the mDia3-EB1-APC and Ndc80 complexes. Therefore, we speculate that EB1 may be able to relay its intrinsic tip tracking activity to the core kinetochore-microtubule attachment apparatus through its interaction with mDia3 upon spindle microtubule capture (). This is a tempting idea because: (1) Depletion of mDia3,Citation14 results in chromosome misalignment phenotype, reminiscent of depletion of the Ndc80 complex;Citation25 (2) similar to what has been shown for Ndc80,Citation4 mDia3 microtubule binding and stabilization activity is also regulated by Aurora B phosphorylation;Citation14 and (3) Spc105, a component of the conserved Ndc80 complex in yeast, can rescue mutations in the EB1 ortholog mal3.Citation26 Clearly, determining whether and how the mDia3-EB1-APC complex interacts with the Ndc80 complex and affects Ndc80 microtubule association will be key to understanding how end-on kinetohcores track plus ends of growing microtubules.
Figures and Tables
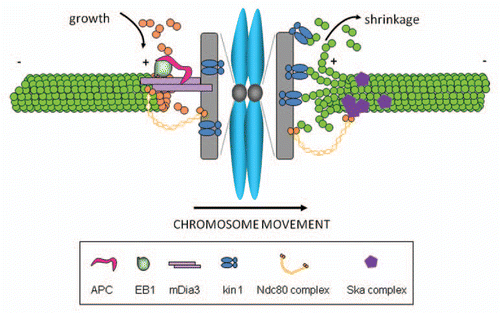
Figure 1 A Model for kinetochores tracking spindle microtubule plus-ends. During metaphase chromosome oscillation, the leading and trailing kinetochores in a sister kinetochore pair have two distinct stably attached microtubule populations: the trailing kinetochore is attached to growing microtubules, whereas the leading kinetochore retains its attachment to shrinking microtubules. The Ndc80 complex is a central component in stabilizing end-on kinetochore microtubule attachment. Microtubule plus-end tracking proteins, EB1 and APC, track plus ends of growing microtubules. The interaction between EB1-APC and formin mDia3, a novel kinetochore protein, may mediate continuous Ndc80 tip tracking with growing microtubules, either directly or through other unknown linker proteins. The Ska complex is able to mediate a cargo association with depolymerizing microtubule plus ends in vitro.Citation27 Whether the Ska complex is able to interact with Ndc80 and increase the stability of connection between Ndc80 and shrinking microtubule ends is not clear.
Acknowledgments
We are grateful to Y. Guo and S. Ahmad for critically reading the manuscript. Y. Mao is an Irma T. Hirschl Career Scientist.
Addendum to:
References
- Cleveland DW, Mao Y, Sullivan KF. Centromeres and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell 2003; 112:407 - 421
- Cheeseman IM, Desai A. Molecular architecture of the kinetochore-microtubule interface. Nat Rev Mol Cell Biol 2008; 9:33 - 46
- Joglekar AP, Bloom KS, Salmon ED. Mechanisms of force generation by end-on kinetochore-microtubule attachments. Curr Opin Cell Biol 2010; 22:57 - 67
- Cheeseman IM, Chappie JS, Wilson-Kubalek EM, Desai A. The conserved KMN network constitutes the core microtubule-binding site of the kinetochore. Cell 2006; 127:983 - 997
- Ciferri C, Pasqualato S, Screpanti E, Varetti G, Santaguida S, Dos Reis G, et al. Implications for kinetochore-microtubule attachment from the structure of an engineered Ndc80 complex. Cell 2008; 133:427 - 439
- Powers AF, Franck AD, Gestaut DR, Cooper J, Gracyzk B, Wei RR, et al. The Ndc80 kinetochore complex forms load-bearing attachments to dynamic microtubule tips via biased diffusion. Cell 2009; 136:865 - 875
- Lampert F, Hornung P, Westermann S. The Dam1 complex confers microtubule plus end-tracking activity to the Ndc80 kinetochore complex. J Cell Biol 2010; 189:641 - 649
- Tirnauer JS, Canman JC, Salmon ED, Mitchison TJ. EB1 targets to kinetochores with attached, polymerizing microtubules. Mol Biol Cell 2002; 13:4308 - 4316
- Draviam VM, Shapiro I, Aldridge B, Sorger PK. Misorientation and reduced stretching of aligned sister kinetochores promote chromosome missegregation in EB1- or APC-depleted cells. EMBO J 2006; 25:2814 - 2827
- Green RA, Wollman R, Kaplan KB. APC and EB1 function together in mitosis to regulate spindle dynamics and chromosome alignment. Mol Biol Cell 2005; 16:4609 - 4622
- Zhang J, Ahmad S, Mao Y. BubR1 and APC/EB1 cooperate to maintain metaphase chromosome alignment. J Cell Biol 2007; 178:773 - 784
- Mimori-Kiyosue Y, Shiina N, Tsukita S. The dynamic behavior of the APC-binding protein EB1 on the distal ends of microtubules. Curr Biol 2000; 10:865 - 868
- Slep KC, Vale RD. Structural basis of microtubule plus end tracking by XMAP215, CLIP-170 and EB1. Mol Cell 2007; 27:976 - 991
- Cheng L, Zhang J, Ahmad S, Rozier L, Yu H, Deng H, et al. Aurora B regulates formin mDia3 in achieving metaphase chromosome alignment. Dev Cell 2011; 20:342 - 352
- Bartolini F, Gundersen GG. Formins and microtubules. Biochim Biophys Acta 2009;
- Kohno H, Tanaka K, Mino A, Umikawa M, Imamura H, Fujiwara T, et al. Bni1p implicated in cytoskeletal control is a putative target of Rho1p small GTP binding protein in Saccharomyces cerevisiae. EMBO J 1996; 15:6060 - 6068
- Lee L, Klee SK, Evangelista M, Boone C, Pellman D. Control of mitotic spindle position by the Saccharomyces cerevisiae formin Bni1p. J Cell Biol 1999; 144:947 - 961
- Adames NR, Cooper JA. Microtubule interactions with the cell cortex causing nuclear movements in Saccharomyces cerevisiae. J Cell Biol 2000; 149:863 - 874
- Bloom K. It's a kar9ochore to capture microtubules. Nat Cell Biol 2000; 2:96 - 98
- Kusch J, Liakopoulos D, Barral Y. Spindle asymmetry: a compass for the cell. Trends Cell Biol 2003; 13:562 - 569
- Schuyler SC, Pellman D. Search, capture and signal: games microtubules and centrosomes play. J Cell Sci 2001; 114:247 - 255
- Wen Y, Eng CH, Schmoranzer J, Cabrera-Poch N, Morris EJ, Chen M, et al. EB1 and APC bind to mDia to stabilize microtubules downstream of Rho and promote cell migration. Nat Cell Biol 2004; 6:820 - 830
- Kaplan KB, Burds AA, Swedlow JR, Bekir SS, Sorger PK, Nathke IS. A role for the Adenomatous Polyposis Coli protein in chromosome segregation. Nat Cell Biol 2001; 3:429 - 432
- Yasuda S, Oceguera-Yanez F, Kato T, Okamoto M, Yonemura S, Terada Y, et al. Cdc42 and mDia3 regulate microtubule attachment to kinetochores. Nature 2004; 428:767 - 771
- DeLuca JG, Gall WE, Ciferri C, Cimini D, Musacchio A, Salmon ED. Kinetochore microtubule dynamics and attachment stability are regulated by Hec1. Cell 2006; 127:969 - 982
- Kerres A, Vietmeier-Decker C, Ortiz J, Karig I, Beuter C, Hegemann J, et al. The fission yeast kinetochore component Spc7 associates with the EB1 family member Mal3 and is required for kinetochore-spindle association. Mol Biol Cell 2004; 15:5255 - 5267
- Welburn JP, Grishchuk EL, Backer CB, Wilson-Kubalek EM, Yates JR 3rd, Cheeseman IM. The human kinetochore Ska1 complex facilitates microtubule depolymerization-coupled motility. Dev Cell 2009; 16:374 - 385