Abstract
During regeneration, lost structures are rebuilt and perfectly integrated within the remaining non-injured tissues. This fascinating process captured the attention of one of the founders of modern genetics, T. H. Morgan. He was particularly interested in understanding regeneration in freshwater planarians, which can regenerate a whole animal from a small piece of their bodies. He performed numerous experiments to understand how polarity is re-established such that an anterior-facing wound regenerates a head whereas a posterior-facing wound regenerates a tail. However, it has not been until more than 100 years later that the molecules required to determine axial polarity have been identified. Several studies have now shown that the Wnt/β-catenin and Hedgehog pathways are required for anteroposterior axis specification, whereas the establishment of the planarian dorsoventral (DV) axis relies on the Bone Morphogenetic Protein (BMP) pathway. Two recent papers have now uncovered additional conserved (anti-dorsalizing morphogenetic protein) and novel (noggin-like genes) elements that regulate planarian DV axis regeneration. Here, we summarize those results and present new data and hypotheses to explain the role that noggin-like genes might play.
Similarly to its function during invertebrate embryonic development, the BMP pathway is required to specify and maintain dorsal identity during planarian regeneration and homeostasis.Citation1–Citation3 Thus, loss-of-function of the ligand bmp or the intracellular elements smad1 or smad4-1 results in partial ventralization; an ectopic central nervous system (CNS) and a mouth opening differentiate dorsally, expression of some dorsal markers disappears and dorsal epithelial cilia adopt a ventral-like pattern.Citation1–Citation4 Silencing of the BMP pathway does not result in fully ventralized animals, however, as new DV boundaries appear dorsallyCitation1,Citation2 and some dorsal structures and cell types such as the eyes and the nanos-positive presumptive male primordial germ cellsCitation5–Citation7 do not disappear but rather differentiate deeper inside the mesenchyme.Citation1,Citation2 The resulting phenotype appears to involve two ventral sides connected by an intermediate dorsal region. On the other hand, the silencing of planarian noggins, well-known antagonists of BMP signaling, yields complementary dorsalizing phenotypes in which ectopic outgrowths expressing dorsal markers and differentiating eyes eventually develop in the anterior ventral region.Citation4
Although in vertebrates BMP signaling promotes ventral fates, during Xenopus development both dorsal and ventral signaling centers serve as sources of BMPs and their modulators (reviewed in ref. Citation8–Citation10). For instance, the ligand ADMP (anti-dorsalizing morphogenetic protein, a member of the BMP family) and antagonists such as Chordin and Noggin are secreted by the dorsal center, whereas BMP4 and BMP7, and other antagonists are secreted ventrally. Together, these proteins configure a complex self-regulatory circuit that restricts BMPs and ADMP signaling to the ventral region of the embryo.Citation8,Citation9,Citation11 Thus, the dorsal side has the lowest BMP signaling and the ventral side the highest.
Recently, our group and others have reported that a BMP/ADMP regulatory circuit directs re-establishment of DV polarity in invertebrates.Citation4,Citation12 As expected, planarian bmp and admp-1 show complementary expression patterns along the dorsal and ventral midlines, respectively.Citation1,Citation3,Citation4,Citation12,Citation13 As in Xenopus, activation of BMP signaling inhibits admp-1 expression whereas ADMP activity promotes bmp expression.Citation12 Further evidence for this BMP/ADMP circuit comes from the observation that co-silencing of bmp and admp-1 strengthens bmp loss-of-function phenotypes.Citation4,Citation12 Despite these similarities between planarians and Xenopus, simultaneous inhibition of bmps and admp homologs in planarians does not lead to the catastrophic loss of DV polarity seen in Xenopus,Citation11 suggesting that additional molecules may play a role in planarian DV patterning.Citation4,Citation12
The planarian BMP/ADMP circuit seems to be regulated by canonical antagonists of the noggin family as well as by novel noggin-like genes (nlg).Citation4,Citation14 The unexpected activity of this family of novel regulatory elements adds a new step in the complex regulation of DV axis establishment. In contrast to noggins, the silencing of planarian noggin-like gene 8 (nlg8) yields similar phenotypes to those obtained in bmp knockdowns, with ectopic dorsal CNS differentiation. Unlike bmp silencing, however, nlg8 knockdown does not cause thickening, DV border duplication or disappearance of dorsal markers, and although cilia are reduced in the dorsal lateral regions, ventralization of the stereotypical pattern of dorsal cilia does not occur.Citation4 In addition, whereas bmp or smad1 loss-of-function results in a posterior-to-anterior differentiation of ectopic dorsal nerve cords (connected to the ventral nerve cords at the tail end), after nlg8 silencing the ectopic CNS appears preferentially in the anterior region and, in some cases, seems to connect with a dorsal expansion of the ventral cephalic ganglia.Citation1,Citation4 Finally, although nlg8 silencing produces essentially neural phenotypes, the fact that its co-silencing enhances both the dorsalization obtained after noggins loss-of-function and the ventralization produced by the inhibition of BMP signalingCitation4 suggests that this novel element may be involved in DV patterning. Therefore, nlg8 appears to have a dorsalizing or ventralizing effect depending on the context, as has been shown for some antagonists of the BMP pathway in other organisms (reviewed in ref. Citation9).
In organisms as distant as Drosophila and Xenopus, the establishment of the DV axis and the specification of neural and ectodermal territories are closely related processes.Citation10,Citation15 During early development, the BMP pathway acts as a potent antineurogenic factor and, consequently, neural territories are specified in the region of lowest BMP signaling. Accordingly, the overactivation or inhibition of the BMP pathway in Xenopus embryos ventralizes (expansion of the ectodermal territory) or dorsalizes (expansion and/or duplication of neural territories) those embryos, respectively.Citation11 Whereas the overexpression of planarian nlg8 in Xenopus embryos results in the ventralization of the embryo and reduces the expression domain of the neural marker sox2, its silencing in planarians does not severely affect the DV axis but mainly promotes ectopic neural differentiation.Citation4 On this basis, we hypothesize that, in planarians, nlg8 knockdowns reduce a threshold of BMP activity required for its antineurogenic role without further affecting DV patterning ().
Irrespective of the effect of nlg8 silencing on the level of BMP signaling, nlg8 or bmp silencing always generates an ectopic dorsal CNS in a position mirroring that of the ventral nervous system.Citation1–Citation4 Throughout the evolution of the Platyhelminthes, there seems to have been a reduction in the number of nerve cords, as some groups present several pairs of nerve cords both ventrally and dorsally.Citation16 Therefore, an evolutionary explanation for the position of the ectopic CNS could be that only specific dorsal regions are competent to differentiate such tissues. Such a possibility is also consistent with the observation that strictly asexual planarians have two dorsal rows of cells expressing the germ cell marker nanos in a pattern reminiscent of the position of the testis in sexual animals.Citation5–Citation7
Remarkably, noggin-like genes are evolutionarily conserved and can be found from cnidarians to vertebrates.Citation4 In agreement with the functional studies in planarians, the overexpression of the Xenopus noggin-like mRNA into Xenopus embryos phenocopies bmp overexpression and produces ventralized embryos. However, unexpectedly, this ventralization does not occur due to increased SMAD1/5/8 phosphorylation (a readout of BMP signaling activation).Citation4 Thus, the explanation for the opposing functions of noggins and noggin-likes remains unclear.Citation4 One area that deserves further attention is the correlation between structure and function. Noggin-like genes lack a heparin-binding domain in their N-terminal region and bear an amino acidic insertion within the conserved noggin domain.Citation14 Interestingly, the removal of the amino-acid insertion from Xenopus nlg yields dorsalized embryos.Citation4 These results indicate a relevant function for the insertion, perhaps via an effect on binding to BMP or other factors involved in DV patterning (). Alternatively, the absence of a heparinbinding domain in noggin-like genes could increase their capacity to diffuse through the extracellular matrix.
Finally, these data suggest that the phenotypes obtained after silencing or overexpression of nlg could be explained as the result of either noggin-like modulating BMP activity through a pSMAD1/5/8-independent mechanism or an effect on pathways other than BMP. In order to clarify the mechanism of action of noggin-like and its exact relationship with BMP signaling, biochemical studies will be required to asses the interactions between this novel family and the other elements of the pathway.
Figures and Tables
Figure 1 Graphical representation of a proposed gradient of BMP signaling in the dorsal region of the planarian and its hypothetical reduction after different RNAi knockdowns. After nlg8 silencing the decrease in BMP signaling around the midline would not be sufficient to affect DV patterning. However, in the lateral regions, the reduction of BMP activity would eliminate the antineurogenic effect of the pathway and allow the differentiation of an ectopic CNS (arrows). Only after bmp silencing, alone or in combination, would the reduction in the level of BMP activity around the midline result in ventralized planarians.
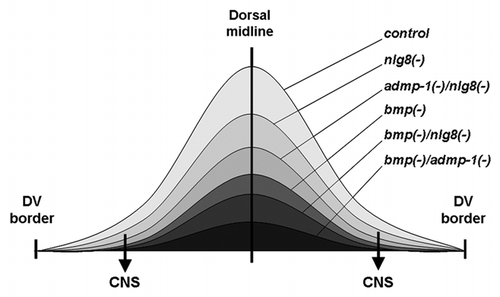
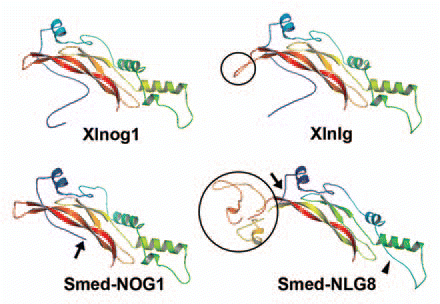
Figure 2 Comparison of the predicted protein structures of noggin and noggin-like proteins. Homology-based protein structures of Xenopus noggin1 (Xlnog1), planarian NOGGIN1 (Smed-NOG1), Xenopus noggin-like (Xlnlg) and planarian NOGGIN-LIKE 8 (Smed-NLG8) constructed by the Swissmodel programCitation17 and further modified using PyMOL software (DeLano Scientific LLC, pymol.sourceforge.net/) are shown. The structure of planarian NOGGIN shows a high degree of similarity to the Xenopus noggin1 homolog, although its BMP-binding domain is shorter (arrow). With the exception of the insertion (black circles), the predicted structures of the noggin-like homologs resemble those of noggins. Although the length of the amino-acid insertion varies between species,Citation4 its presence could probably modify the binding capacities of noggin-like homologs. Note that a helix is absent in Smed-NLG8 (arrowhead). Accession numbers: AAT91717, ABV04323, NP_001089147, ACO06233.
Acknowledgments
We thank Iain Pattern for advice on English style in a version of the manuscript. M.D.M. was funded by an FPU fellowship from the Ministerio de Educación y Ciencia (Spain). F.C. is a Ramón y Cajal researcher (Ministerio de Ciencia e Innovación, Spain). This work was funded by grants BFU2008-01544 (MICINN) to E.S., grant BFU2008-00710 (MICINN) to F.C., and grant 2009SGR1018 (AGAUR, Generalitat de Catalunya) to E.S. and F.C.
Addendum to:
References
- Molina MD, Saló E, Cebrià F. The BMP pathway is essential for re-specification and maintenance of the dorsoventral axis in regenerating and intact planarians. Dev Biol 2007; 311:79 - 94
- Orii H, Watanabe K. Bone morphogenetic protein is required for dorso-ventral patterning in the planarian Dugesia japonica. Dev Growth Differ 2007; 49:345 - 349
- Reddien PW, Bermange AL, Kicza AM, Sanchez Alvarado A. BMP signaling regulates the dorsal planarian midline and is needed for asymmetric regeneration. Development 2007; 134:4043 - 4051
- Molina MD, Neto A, Maeso I, Gómez-Skarmeta JL, Saló E, Cebrià F. Noggin and noggin-like genes control dorsoventral axis regeneration in planarians. Curr Biol 2011; 21:300 - 305
- Handberg-Thorsager M, Saló E. The planarian nanos-like gene Smednos is expressed in germline and eye precursor cells during development and regeneration. Dev Genes Evol 2007; 217:403 - 411
- Sato K, Shibata N, Orii H, Amikura R, Sakurai T, Agata K, et al. Identification and origin of the germline stem cells as revealed by the expression of nanos-related gene in planarians. Dev Growth Differ 2006; 48:615 - 628
- Wang Y, Zayas RM, Guo T, Newmark PA. nanos function is essential for development and regeneration of planarian germ cells. Proc Natl Acad Sci USA 2007; 104:5901 - 5906
- De Robertis EM. Spemann's organizer and the self-regulation of embryonic fields. Mech Dev 2009; 126:925 - 941
- Plouhinec JL, De Robertis EM. Systems biology of the self-regulating morphogenetic gradient of the Xenopus gastrula. Cold Spring Harb Perspect Biol 2009; 1:001701
- De Robertis EM, Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Annu Rev Cell Dev Biol 2004; 20:285 - 308
- Reversade B, De Robertis EM. Regulation of ADMP and BMP2/4/7 at opposite embryonic poles generates a self-regulating morphogenetic field. Cell 2005; 123:1147 - 1160
- Gaviño MA, Reddien PW. A Bmp/Admp regulatory circuit controls maintenance and regeneration of dorsal-ventral polarity in planarians. Curr Biol 2011; 21:294 - 299
- Orii H, Kato K, Agata K, Watanabe K. Molecular cloning of Bone Morphogenetic Protein (BMP) gene from the planarian Dugesia japonica. Zool Sci 1998; 15:871 - 877
- Molina MD, Salo E, Cebria F. Expression pattern of the expanded noggin gene family in the planarian Schmidtea mediterranea. Gene Expr Patterns 2009; 9:246 - 253
- Lynch JA, Roth S. The evolution of dorsal-ventral patterning mechanisms in insects. Genes Dev 2011; 25:107 - 118
- Reuter M, Gustafsson MK. The flatworm nervous system: pattern and phylogeny. Exs 1995; 72:25 - 59
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics 2006; 22:195 - 201