Abstract
Measurement of the activity of neuronal ensembles is an essential step to understand how the neuronal network is organized and functioning. Electrical excitation of neurons causes calcium influx via voltage-gated calcium ion channels, which can be monitored by calcium imaging using fluorescent calcium probes. DNA-encoded calcium indicators (DECIs) such as cameleon and GCaMP have been developed to specifically label a subpopulation of neurons. However, in many cases, DECIs that had been developed and tested in vitro did not always show expected performance in vivo. It is necessary to increase its sensitivity and also to adjust its dynamic range to the physiological conditions. In our recent study, we developed an improved version of GCaMP and tested its performance in vivo using transgenic zebrafish. By combining the new GCaMP with targeted gene expression via the Gal4FF-UAS system, we successfully imaged the activity of the spinal motor circuit during spontaneous contractions of zebrafish larvae. Further we report here that heptanol, a gap junction blocker, could alter the spatiotemporal activation pattern of the motor circuit. Thus, we demonstrate that calcium imaging with GCaMP is powerful to analyze neuronal activities under normal and pharmacologically perturbed conditions.
To understand how animal behaviors are controlled by neuronal networks, it is important to analyze the activity of multiple neurons that form a functional unit to perform a particular task. Electrophysiological recording with multiple electrodes is one way to detect such activities.Citation1 However, neurons that can be analyzed are constrained by the configuration of the recording device. An alternative way is to monitor calcium ion influx through voltage-gated calcium ion channels instead of monitoring the voltage itself.Citation2 Fluorescent calcium indicator dyes have been used successfully for imaging of multiple neurons in various systems.Citation3,Citation4 In this approach, the fluorescent dyes are introduced into neurons by injection or by bulk-loading of an acetoxymethyl ester form. Although the bulk-loading method is suited for labeling of multiple neurons, it is difficult to extract specific calcium signals from individual neurons when a mass of densely packed neurons are labeled. To circumvent these problems, DNA-encoded calcium indicators (DECIs) were developed.Citation5,Citation6 These enabled genetic introduction of a calcium indicator to a specific neuronal type by controlling its expression under the control of a cell-type specific promoter. However, in spite of high expectations, the performance of DECIs in vivo has not been satisfactory in most cases probably because their calcium binding kinetics was not optimized for physiological conditions in vivo.Citation7
Recently, we developed an improved version of GCaMP, GCaMP-HS.Citation8 We showed that GCaMP-HS has a higher refolding activity, increased expression levels in cultured cells and a higher affinity to calcium ions. Then we aimed to test its performance in vivo by constructing transgenic zebrafish that expressed GCaMP-HS with the Gal4FF-UAS system.Citation9 First, we generated UAS:GCaMPHS transgenic fish carrying the GCaMP-HS gene downstream of the Gal4 recognition sequence UAS. Then we conducted a large-scale genetic screen using gene trap constructs to identify transgenic fish expressing the modified yeast transcription factor Gal4FF in specific cell types. We found that the SAIG213A fish line expressed Gal4FF strongly in a specific subset of primary motor neurons called CaP.Citation8 The CaP motor neurons are generated in the ventral spinal cord. One pair of CaP neurons locates on the left and the right side of one spinal segment and extends their axons to the ventral trunk muscles.Citation10
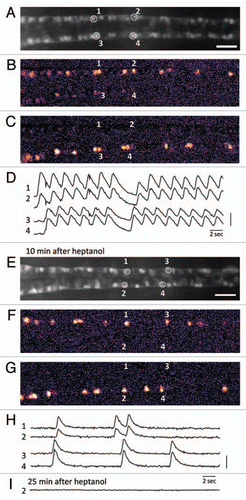
The zebrafish motor system develops quickly after fertilization. Embryos exhibit spontaneous contractions on the left and right trunk muscles in a regularly alternating manner after 17 h post-fertilization (hpf).Citation11 To image the activity of the motor neurons during this behavior, we crossed the UAS:GCaMPHS fish with the SAIG213A fish and obtained the SAIG213A;UAS:GCaMPHS double transgenic embryos that expressed GCaMP-HS in the CaP motor neurons (). Then we performed calcium imaging using embryos embedded in agarose, and found that the fluorescence changes of GCaMP-HS were perfectly matched with actual muscle contractions. More detailed calcium imaging was conducted by using a double transgenic embryo immobilized with a neuromuscular junction blocker, D-tubocurarine and synchronized activation of ipsilateral CaP motor neurons and alternated activation of contralateral CaP neurons were demonstratedCitation8 ().
Previous studies suggested that the coiling behavior in the early developmental stages is mediated mainly by gap junction but not by synaptic transmission.Citation12 We tested the effect of a cell-permeable gap junction blocker, heptanol, in our calcium imaging system (). When the SAIGFF213A;UAS:GCaMPHS double transgenic embryos embedded in agarose were exposed to 1 mM heptanol, they were paralyzed 5–10 min after the exposure. We conducted calcium imaging 10 min after the start of the heptanol treatment, and found that, although the embryo was paralyzed, calcium transients could be detected ( and G). Surprisingly, the synchronized activation of the ipsilateral CaP neurons was observed while regularly alternated activation of contralateral CaP neurons was not detected, revealing robustness of a system to generate the synchronization (). It is interesting to note that activation of contralateral CaP neurons was in most cases coupled with intervals of 0.79 ± 0.22 sec that were slightly shorter than that observed in the heptanol-untreated embryos (1.17 ± 0.13 sec), suggesting that a system that induces activation of contralateral neurons still retained its residual activity (). Calcium transients were completely abolished by 25 min ().
We demonstrated that the improved GCaMP technology combined with the Gal4FF-UAS system in zebrafish is powerful to image activities of a specific neuronal circuit in a living vertebrate. Since the GCaMP-HS can be expressed in any Gal4FF expressing neurons, imaging of neuronal circuits other than the motor circuit will be possible. In this study, we also showed that a gap junction blocker heptanol prohibits the activity of motor neurons as shown in the previous study by an electrophysiological approach.Citation12 Further, we could detect the activity of the motor circuit in transition stages created by the heptanol treatment. Thus, we propose that our calcium imaging system is applicable to pharmacological studies in which effects of a small molecule on neuronal activities are analyzed. In the future, we expect that we can apply this method to imaging of neuronal circuits in the brain that are functioning during cognition, learning and memory.
Figures and Tables
Figure 1 Calcium imaging of the motor circuit in the zebrafish spinal cord during spontaneous contractions. (A) Expression of GCaMP-HS in the CaP motor neurons in the spinal cord. A dorsal view of the SAIG213A;UA S:GCaMPHS double transgenic embryo embedded in 2% low-melting agarose under a fluorescence microscope and treated with a neuromuscular junction blocker, D-tubocurarine. CaP neurons #1–#4 were used as ROIs (regions of interest). Anterior to the left. Scale bar: 200 µm. (B and C) Calcium signals of the CaP motor neurons with pseudocolors (see also www.youtube.com/watch?v=6y44uxrh7z4). (B) The CaP motor neurons on the right side including ROI-1 and -2 showed increased fluorescence. (C) The CaP motor neurons on the left side including ROI-3 and -4 showed increased fluorescence. (D) The fluorescence changes in the selected CaP motor neurons. 1 and 2 (3 and 4) are activated synchronously, and the right (1 and 2) and left (3 and 4) neurons are activated alternately. A vertical bar indicates (F-resting F)/resting F = 50%. (E) A dorsal view of the SAIG213A;UA S:GCaMPHS double transgenic embryo embedded in 2% low-melting agarose under a fluorescence microscope and treated with a gap junction blocker, heptanol, for 10 min. Anterior to the left. Scale bar: 200 µm. (F and G) Calcium imaging of the CaP motor neurons in the presence of heptanol with pseudocolors (see also www.youtube.com/watch?v=3xhw9D35H5w). (F) The CaP motor neurons on the right side including ROI-1 and -2 showed increased fluorescence. (G) The CaP motor neurons on the left side including ROI-3 and -4 showed increased fluorescence. (H) The fluorescence changes in the selected CaP motor neurons. Synchronized activation is still observed. A vertical bar indicates (F-resting F)/resting F = 100%. (I) The fluorescence changes are not detected 25 min after the heptanol treatment.
Acknowledgements
We thank A. Ito, Y. Kanebako, N. Mouri, M. Mizushina and M. Suzuki for fish room works, H. Takakubo for technical assistance, the Mitsubishi Foundation (to A.M.) and the National BioResource Project and grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
Addendum to:
References
- Harris CA, Passaro PA, Kemenes I, Kemenes G, O'Shea M. Sensory driven multi-neuronal activity and associative learning monitored in an intact CNS on a multielectrode array. J Neurosci Methods 2010; 186:171 - 178
- Lipscombe D, Madison DV, Poenie M, Reuter H, Tsien RY, Tsien RW. Spatial distribution of calcium channels and cytosolic calcium transients in growth cones and cell bodies of sympathetic neurons. Proc Natl Acad Sci USA 1988; 85:2398 - 2402
- Ikegaya Y, Aaron G, Cossart R, Aronov D, Lampl I, Ferster D, et al. Synfire chains and cortical songs: temporal modules of cortical activity. Science 2004; 304:559 - 564
- Fetcho JR, O'Malley DM. Visualization of active neural circuitry in the spinal cord of intact zebrafish. J Neurophysiol 1995; 73:399 - 406
- Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol 2001; 19:137 - 141
- Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997; 388:882 - 887
- Hendel T, Mank M, Schnell B, Griesbeck O, Borst A, Reiff DF. Fluorescence changes of genetic calcium indicators and OGB-1 correlated with neural activity and calcium in vivo and in vitro. J Neurosci 2008; 28:7399 - 7411
- Muto A, Ohkura M, Kotani T, Higashijima S, Nakai J, Kawakami K. Genetic visualization with an improved GCaMP calcium indicator reveals spatiotemporal activation of the spinal motor neurons in zebrafish. Proc Natl Acad Sci USA 2011; 108:5425 - 5430
- Asakawa K, Suster ML, Mizusawa K, Nagayoshi S, Kotani T, Urasaki A, et al. Genetic dissection of neural circuits by Tol2 transposon-mediated Gal4 gene and enhancer trapping in zebrafish. Proc Natl Acad Sci USA 2008; 105:1255 - 1260
- Myers PZ, Eisen JS, Westerfield M. Development and axonal outgrowth of identified motoneurons in the zebrafish. J Neurosci 1986; 6:2278 - 2289
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol 1998; 37:622 - 632
- Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron 2001; 31:1035 - 1046