Abstract
The E3-ubiquitinligase MYCBP2 regulates neuronal growth, synaptogenesis, and synaptic plasticity by modulating several signalling pathways including the p38 MAPK signaling cascade. We found that loss of MYCBP2 in peripheral sensory neurons inhibits the internalization of transient receptor potential vanilloid receptor 1 (TRPV1) in a p38 MAPK-dependent manner. This prevented desensitization of activity-induced calcium increases and prolongs formalin-induced thermal hyperalgesia in mice. Besides its function in pain perception TRPV1 is also involved in the regulation of neuronal growth. Therefore, the observed effect of MYCBP2 on TRPV1 internalization could be part of the mechanisms underlying its well documented regulatory role in neuronal growth. The clarification of the mechanism is important for the understanding of the different MYCBP2-functions in diverse neuronal subpopulations and species.
The E3-ubiquitin ligase MYC binding protein 2 (MYCBP2) (1) is a giant protein of 510 kDa which regulates several signaling pathways and physiological processes through a multitude of protein binding sites. MYCBP2 has originally been identified as Protein Associated with Myc (PAM) and orthologs have been described in mouse as Phr1, in Zebrafish as Esrom, in Drosophila as Highwire and in C. elegans as RPM-1. While MYCBP2 mRNA is found in nearly all human tissues, its expression is exceptionally high in peripheral and central neurons.Citation1–Citation3 MYCBP2 is upregulated shortly after birth in the cerebellum and hippocampus, a time representing the major synaptogenic period in these structures and correlating with the regulation of neuronal growth by MYCBP2.Citation2 MYCBP2 has been demonstrated to influence neuronal outgrowth and synaptogenesis by regulating the cAMP,Citation4,Citation5 Smad4,Citation6 mTORCitation17,Citation8 and p38 MAPK-signaling pathways.Citation9
Independently of its well documented role as negative regulator of neuronal growth,Citation6–Citation9 MYCBP2 also modulates synaptic functions of adult animals. In this regard it has previously been shown that MYCBP2 decreases spinal nociceptive processing by inhibition of adenylyl cyclases, the enzyme which catalyzes the conversion of ATP to cAMP.Citation3 It is well known that elevated cAMP levels increase neuronal excitability mainly through protein kinase A (PKA)-dependent phosphorylation of ion channels as well as PKA- and cAMP-response-element-binding protein (CREB)-mediated changes in gene expression.Citation10 Accordingly, downregulation of MYCBP2 in the spinal cord has been shown to cause an increase in the cAMP-synthesis and, therefore, nociceptive behavior.Citation3
Regulation of Receptor Trafficking by MYCBP2
MAPK kinase kinase 12 (MAP3K12, also known as dual leucine zipper bearing kinase, DLK is an ubiquitylation target of MYCBP2.Citation9 MAP3K12 activates the p38 MAPK-pathway. Interestingly, p38 MAPK regulates the TRPV1-translation, TRPV1 axonal transport, TRPV1-recruitment to the nociceptorCitation11 and the production of important proinflammatory cytokines like TNFα or IL-1 Citation12 making p38 MAPK an important factor for sensibilization procedures of nociceptive neurons. In this regard it was not surprising that loss of MYCBP2 in peripheral sensory neurons caused an increased nociceptive behavior in an animal model for inflammatory pain. We found that MYCBP2-deficiency caused a constitutive p38 MAPK activation which, surprisingly, prevented activity-induced internalization of the transient receptor potential vanilloid receptor 1 (TRPV1) leading to the p38 MAPK-mediated prolonged thermal hyperalgesia.
The observed inhibition of TRPV1-internalization by loss of MYCBP2 contrasts the previously described role of p38 MAPK in receptor internalization in other mammalian systems.Citation13–Citation15 Even though an inhibition of endocytosis would augment the increased translation and peripheral transport of TRPV1 by activated p38 MAPK. However, it was demonstrated that p38 MAPK activation is necessary for µ-opioid receptor endocytosis and is sufficient to trigger constitutive internalization of µ-opioid receptor in the absence of agonists.Citation14 These contrasting findings suggest that p38 MAPK is able to fulfil different roles in receptor trafficking. This assumption is supported by the finding that phosphorylation of epidermal growth factor (EGF)-receptor between amino acids 1002 and 1022 mediates stress-induced internalizationCitation15 while EGFR phosphorylation at serine 1046 and 1047 by p38 MAPK mediates ubiquitylation and degradation of already internalized EGF receptor but not internalization itself.Citation16,Citation17 Therefore it is not completely surprising that the loss of MYCBP-ortholog RPM-1 facilitates AMPA receptor internalization in C. elegans.Citation18 Additionally it should be noted that RPM-1-mediated increased AMPA receptor internalization is found in central interneurons, while the prevention of MYCBP2-mediated TRPV-1 internalization was observed in peripheral sensory neurons ().
Notably, besides the functional role of TRPV1 in nociceptive processing this receptor is also involved in calcium-mediated neuronal growth.Citation19,Citation20 Therefore, it can be hypothesized that the described inhibition of TRPV1 internalization by the loss of MYCBP2 might be one of the mechanism by which MYCBP2 regulates neuronal growth.
Neuron-Specific MYCBP2 Functions
The possibility that MYCBP2 fulfils diverse functions in the different neuronal subpopulations has already been raised previously. Grill et al. hypothesized that two parallel MYCBP2-mediated signaling pathways regulate neuronal growth in C. elegans.Citation21 This assumption was partly based on the findings that loss of the MYCBP2-ortholog RPM-1 in motor neurons causes less synapses with disorganized structuresCitation9,Citation22 while RPM-1-deficiency in mechanosensory neurons leads to instable synapse branching and prolonged axonal growth.Citation23 Furthermore MYCBP2-mediated growth of cortical axons in mice is MAP3K12-independent while MYCBP2-dependent axonal growth of spinal cord motor neurons and sensory dorsal root ganglion (DRG) neurons was regulated by p38 MAPK-mediated alterations in microtubule stability.Citation24,Citation25
Diverse roles of PAM in specific neurons are also supported by the reported phenotypes of general and conditional MYCBP2 knockout mice (). The ubiquitous deletion of MYCBP2 leads to neuronal dysfunction and lethality in vertebrates while mutations in invertebrates are viable. The general MYCBP2 deletion in mice causes the development of markedly narrower phrenic nerves with fewer axons, resulting in incomplete innervations of the diaphragm. The resulting respiratory failure leads to postnatal lethality in miceCitation24,Citation26 while, due to their physiological differences, invertebrates show motoric disturbances but are still viable.Citation9,Citation27 Specific deletion of MYCBP2 in motor neurons resulted in an phenotype which allowed some mice to be viable pointing to a possible involvement of non-neuronal expressed MYCBP2.Citation24 Fittingly, specific MYCBP2-deletion in sensory neurons did not influence viability.Citation25 However, it remains unclear why conditional MYCBP2 deletion using the Nestin-promotor, leading to a MYCBP2-deficiency in all neuronal and glia cells, causes prenatal lethality.Citation25 Taken together, the data suggest strongly that MYCBP2 fulfils several roles in regulating neuronal functions and future studies should therefore focus on the specific roles of MYCBP2 in the different cell types.
Figures and Tables
Figure 1 Schematic of the MYCBP2/p38MAPK signaling pathway regulating receptor internalizations. MYCBP2 and its ortholog RPM-1 inhibit the p38 MAPK cascade by targeting MAP3K12 for proteosomal degradation. Activated p38 MAPK prevents the internalization of the TRPV1 at nociceptors of mice, while facilitates at central interneurons of C. elegans the internalization of AMPA receptors.
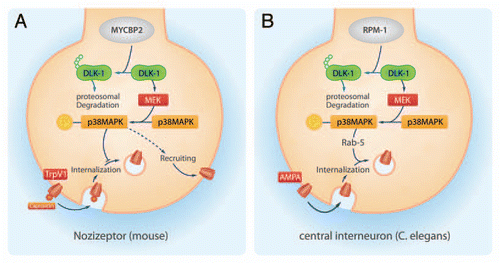
Table 1 Viability of MYCBP2-knockout animals
Addendum to:
References
- Guo Q, Xie J, Dang CV, Liu AT, Bishop JM. Identification of a large Myc-binding protein that contains RCC1-like repeats. Proc Natl Acad Sci USA 1998; 95:9172 - 9177
- Yang H, Scholich K, Poser S, Storm DR, Patel TB, Goldowitz D. Developmental expression of PAM (protein associated with MYC) in the rodent brain. Brain Res Dev Brain Res 2002; 136:35 - 42
- Ehnert C, Tegeder I, Pierre SC, Birod K, Nguyen HV, Schmidtko A, et al. Protein associated with Myc (PAM) is involved in spinal nociceptive processing. J Neurochem 2004; 88:948 - 957
- Scholich K, Pierre SC, Patel TB. Protein associated with Myc (PAM) is a potent inhibitor of adenylyl cyclases. J Biol Chem 2001; 276:47583 - 47589
- Pierre SC, Häusler J, Birod K, Geisslinger G, Scholich K. PAM mediates sustained inhibition of cAMP signaling by sphingosine-1-phosphate. EMBO J 2004; 23:3031 - 3040
- McCabe BD, Hom S, Aberle H, Fetter RD, Marques G, Haerry TE, et al. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 2004; 41:891 - 905
- Maeurer C, Holland S, Pierre S, Potstada W, Scholich K. Sphingosine-1-phosphate induced mTOR-activation is mediated by the E3-ubiquitin ligase PAM. Cell Signal 2009; 21:293 - 300
- Han SWR, Santos TM, Polizzano C, Sabatini BL, Ramesh V. Pam (Protein associated with Myc) functions as an E3 Ubiquitin ligase and regulates TSC/mTOR signaling. Cell Signal 2008; 20:1084 - 1091
- Nakata KAB, Grill B, Goncharov A, Huang X, Chisholm AD, Jin Y. Regulation of a DLK-1 and p38 MAP kinase pathway by the ubiquitin ligase RPM-1 is required for presynaptic development. Cell 2005; 120:407 - 420
- Pierre S, Eschenhagen T, Geisslinger G, Scholich K. Capturing adenylyl cyclases as potential drug targets. Nat Rev Drug Discov 2009; 8:321 - 335
- Ji RRST, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron 2002; 36:57 - 68
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signaling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov 2003; 2:717 - 726
- Huang CC, You JL, Wu MY, Hsu KS. Rap1-induced p38 mitogen-activated protein kinase activation facilitates AMPA receptor trafficking via the GDI.Rab5 complex. Potential role in (S)-3,5-dihydroxyphenylglycene-induced long term depression. J Biol Chem 2004; 279:12286 - 12292
- Mace G, Miaczynska M, Zerial M, Nebreda A R. Phosphorylation of EEA1 by p38 MAP kinase regulates mu opioid receptor endocytosis. EMBO J 2005; 24:3235 - 3246
- Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J 2006; 25:4195 - 4206
- Frey MR, Dise RS, Edelblum KL, Polk D B. p38 kinase regulates epidermal growth factor receptor downregulation and cellular migration. EMBO J 25:5683 - 5692
- Tong J, Taylor P, Peterman SM, Prakash A, Moran MF. Epidermal growth factor receptor phosphorylation sites Ser991 and Tyr998 are implicated in the regulation of receptor endocytosis and phosphorylations at Ser1039 and Thr1041. Mol Cell Proteomics 2009; 8:2131 - 2144
- Park EC, Glodowski DR, Rongo C. The ubiquitin ligase RPM-1 and the p38 MAPK PMK-3 regulate AMPA receptor trafficking. PLoS One 2009; 4:4284
- Goswami C. Structural and functional regulation of growth cone, filopodia and synaptic sites by TRPV1. Commun Integr Biol 2010; 3:614 - 618
- Goswami C, Schmidt H, Hucho F. TRPV1 at nerve endings regulates growth cone morphology and movement through cytoskeleton reorganization. FEBS J 2007; 274:760 - 772
- Grill BBW, Brown HM, Ackley BD, Quadroni M, Jin Y. C. elegans RPM-1 regulates axon termination and synaptogenesis through the Rab GEF GLO-4 and the Rab GTPase GLO-1. Neuron 2007; 55:587 - 601
- Zhen M, Huang X, Bamber B, Jin Y. Regulation of presynaptic terminal organization by C. elegans RPM-1, a putative guanine nucleotide exchanger with a RING-H2 finger domain. Neuron 2000; 26:331 - 343
- Schaefer AM, Hadwiger GD, Nonet ML. rpm-1, a conserved neuronal gene that regulates targeting and synaptogenesis in C. elegans. Neuron 2000; 26:345 - 356
- Bloom AJMB, Sanes JR, DiAntonio A. The requirement for Phr1 in CNS axon tract formation reveals the corticostriatal boundary as a choice point for cortical axons. Genes Dev 2007; 21:2593 - 2606
- Holland S, Coste O, Zhang DD, Pierre SC, Geisslinger G, Scholich K. The ubiquitin ligase MYCBP2 regulates transient receptor potential vanilloid receptor 1 (TRPV1) internalization through inhibition of p38 MAPK signaling. J Biol Chem 2011; 286:3671 - 3680
- Burgess RWPK, Johnson MJ, Roix JJ, Welsh IC, O'Brien TP. Evidence for a conserved function in synapse formation reveals Phr1 as a candidate gene for respiratory failure in newborn mice. Mol Cell Biol 2004; 24:1096 - 1105
- Wan HIDA, Fetter RD, Bergstrom K, Strauss R, Goodman CS. Highwire regulates synaptic growth in Drosophila. Neuron 2000; 26:313 - 329
- D'Souza J, Hendricks M, Le Guyader S, Subburaju S, Grunewald B, Scholich K, Jesuthasan S. Formation of the retinotectal projection requires Esrom, an ortholog of PAM (protein associated with Myc). Development 2005; 132:247 - 256