Abstract
In animal cells, cell division concludes with the separation of two daughter cells during a process called cytokinesis. Abscission, the termination of cytokinesis, is performed through formation of the midbody, a vis-a-vis microtubule (MT)-rich structure bridging the daughter cells. Disassembly of the midbody is the final stage of daughter cell separation and occurs in parallel to membrane fusion in this area. To shed light on this process and to better understand MT organization within the dense area of the midbody structure, an integrative fluorescence microscopy and cryo-electron tomography (cryo-ET) approach was taken.1 These efforts led to a resolving of MT architecture at single-fiber resolution, resulting in a refined model of abscission.
Getting Closer to the Live State by Cryo-Electron Tomography
Electron microscopy images of purified midbodies have provided important information on MTs within these structures.Citation2,Citation3 Still, three-dimensional analysis of the hydrated state has proven challenging, due to technical difficulties.Citation4–Citation6 However, with the introduction of cryo-ET, it is now possible to conduct high-resolution analysis of individual pleiomorphic structures, such as intact cells or even midbodies, in a close-to-native state. Hence, to gain detailed insight into MT organization within the midbody, these structures derived from CHO-cells were vitrified and analyzed by cryo-ET, followed by image-processing analysis.
Organizational Groups of MTs within the Midbody
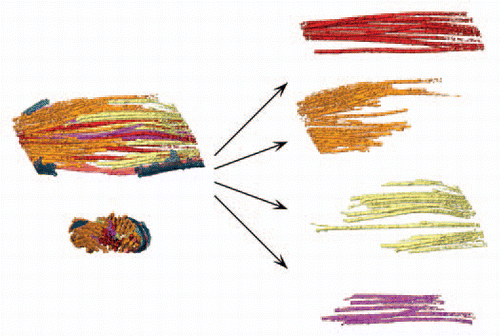
The central domain of the midbody is organized as an anti-parallel array of bundled MTs that form the overlap zone, a highly electron-dense region. Tracking of MTs reveals that within the midbody, these fibers are divided into four morphological groups, depicted in the rendered view presented in . The midbody central region contains MTs traversing the overlap region, termed continuous MTs. A second group, the polar MTs, surrounds the continuous MTs and terminates within the overlap region. This group could be further divided into two subgroups, corresponding to the daughter cell from which they originate ( and orange and yellow fibers, respectively). The fourth group represents minus-end capped MTsCitation1 ( and purple fibers).
To identify MT plus-ends, Elad et al. combined the use of fluorescence microscopy with a construct of GFP fused to the MT end-binding protein 1 (EB1). EB1 was thus localized to four foci within the midbody, namely to the two inner foci that surround the overlap region and to the two outer foci found at both midbody ends. This observation supports the MT architecture observed by cryo-ET,Citation1 with the plus ends of the polar MTs constituting the inner foci, and the plus ends of the continuous MTs constituting the outer foci. Further support for this architecture was provided by EM studies performed on cultured mammalian cell sections.Citation7,Citation8 The number of MTs in vertical sections within the overlap region was found to be 1.5 times the number present in the polar region. Were this region to contain polar MTs alone, a 2:1 ratio would be expected. However, since a third of the MTs are continuous from one polar region to the other, the ratio of the numbers of MTs in the overlap region and reaching either polar region is instead 1.5:1, as predicted by cryo-EM.
MT Assembly within the Midbody
Based on the observation that early telophase MTs present only limited degree of overlap,Citation9 we hypothesized that the continuous bundle is only formed in late telophase. MT bundles that localize in close proximity to the core region polymerize and elongate, whereas the outer, surrounding MTs are instead restricted. Indeed, considerable MT dynamics are observed at the midbody poles. Furthermore, PRC1, an anti-parallel MT cross-linker, was localized to the polar regions only in late but not in early telophase. This supports the notion of elongation of MTs from the overlap region toward the poles and the subsequent formation of new, anti-parallel MT stretches corresponding to the PRC1-binding site.
Breakdown of the Midbody
Midbody breakdown concludes the structural changes that occur within MT bundles that eventually lead to asymmetric division, wherein the remaining structure is pulled towards one of the daughter cells. A recent study revealed the presence of helical-shaped filaments adjacent to the membrane of one midbody pole. These filaments likely play a role in the asymmetric narrowing of the MT bridge spanning the daughter cells.Citation10 During midbody breakdown, a general reduction in the number of MTs is observed. Moreover, before final separation, several changes are detected, according to MT type. The surrounding polar MTs lose their inter-digitating organization and retract from the overlap region. While the continuous MTs maintain their structural morphology during the longer step of the midbody breakdown, their numbers are reduced. Correspondingly, the inner foci region is barely detected when midbodies are in the final collapse stages.
Midbody breakdown and MT reorganization correlate with the termination of cell division. At this point, those MTs in the middle of the midbody that form the dense middle area are separated from the overlap region, while some of the MTs remain intact and persist until the abscission process ends. Accordingly, various overlap region distances are detected, reflecting the progression of the abscission process. The continuous MTs remain at the later stages of the process, while polar MTs are hardly inter-digitated with the opposing polar MTs at this point. Moreover, the inner foci region is barely detected when midbodies are at the final, collapse stages.
As a result of these observations, we propose a model whereby a continuous MT bundle persists until the final stages of cytokinesis. Consequently, the involvement of continuous MTs in cytokinesis is crucial during separation of the two daughter cells, althoughthese MTs may also play a role in cases where daughter cells remain connected for extended periods. Citation11 Future studies combining high-resolution imaging with genetic manipulation will shed light on the precise molecular remodeling that occurs during the final stages of cytokinesis.
Figures and Tables
Acknowledgments
This study was supported by grants from the German-Israeli Cooperation Project (DIP; H.2.2) and by an ERC Starting Grant to O.M. (243047 INCEL).
Addendum to:
References
- Elad N, Abramovitch S, Sabanay H, Medalia O. Microtubule organization in the final stages of cytokinesis as revealed by cryo-electron tomography. J Cell Sci 2011; 124:207 - 215
- Mullins JM, McIntosh JR. Isolation and initial characterization of the mammalian midbody. J Cell Biol 1982; 94:654 - 661
- McIntosh JR, Sisken JE, Chu LK. Structural studies on mitotic spindles isolated from cultured human cells. J Ultrastruct Res 1979; 66:40 - 52
- Ortiz JO, Brandt F, Matias VR, Sennels L, Rappsilber J, Scheres SH, et al. Structure of hibernating ribosomes studied by cryoelectron tomography in vitro and in situ. J Cell Biol 2010; 190:613 - 621
- Baumeister W. From proteomic inventory to architecture. FEBS Lett 2005; 579:933 - 937
- Ben-Harush K, Maimon T, Patla I, Villa E, Medalia O. Visualizing cellular processes at the molecular level by cryo-electron tomography. J Cell Sci 2010; 123:7 - 12
- Brinkley BR, Cartwright J Jr. Ultrastructural analysis of mitotic spindle elongation in mammalian cells in vitro. Direct microtubule counts. J Cell Biol 1971; 50:416 - 431
- McIntosh JR, Landis SC. The distribution of spindle microtubules during mitosis in cultured human cells. J Cell Biol 1971; 49:468 - 497
- Mastronarde DN, McDonald KL, Ding R, McIntosh JR. Interpolar spindle microtubules in PTK cells. J Cell Biol 1993; 123:1475 - 1489
- Guizetti J, Schermelleh L, Mäntler J, Maar S, Poser I, Leonhardt H, et al. Cortical constriction during abscission involves helices of ESCRT-III-dependent filaments. Science 2011; 331:1616 - 1620
- Steigemann P, Gerlich DW. Cytokinetic abscission: cellular dynamics atthe midbody. Trends Cell Biol 2009; 19:606 - 616