Abstract
Drosophila tao, encoding a Ste20 family kinase, was identified as a gene involved in ethanol, cocaine, and nicotine sensitivity. The behavioral phenotypes appear to be caused by defects in the development of the adult brain. Specifically, Drosophila tao functions to promote axon guidance of mushroom body (MB) neurons. The MB is a large structure in the central brain of the fly whose development and function have been well characterized. tao interacts genetically with mutations in the par-1 gene, also encoding a serine-threonine kinase. Since Par-1 has been implicated in the regulation of microtubule dynamics, this suggests that tao regulates the microtubule cytoskeleton in developing MB neurons. Here we discuss these results in light of previous studies that have proposed that Drosophila tao and its mammalian homologues function as a link between the actin and microtubule cytoskeletons, regulating microtubule stability in response to actin signals.
The Tao kinase family is part of the larger Ste20 kinase family, a diverse group of serine-threonine kinases that participate in a variety of signaling pathways.Citation1 The Tao family has three members in mammals (Tao1/Psk2/MARKK, Tao2/Psk1 and Tao3/JIK), but just a single representative in Drosophila melanogaster and C. elegans. The Drosophila tao gene was identified in a screen for behavioral mutants with altered sensitivity to cocaine, and it was subsequently discovered that tao mutations have strong effects on a number of drug-induced behaviors, including sensitivity to nicotine and alcohol.Citation2 These behavioral phenotypes were traced to defects in the development of the adult central brain; specifically, tao's behavioral phenotypes arise from defects in the development of the mushroom body (MB), a brain structure known to be involved in learning and memoryCitation3 and a number of other behaviors such as courtship,Citation4 temperature preference,Citation5 sleepCitation6 and oviposition preference.Citation7
When Drosophila tao mutants were examined by immunohistochemistry with an antibody that stains MB axons, it was found that these mutants lack MB lobes. The cell bodies of the roughly 2,000 MB neurons lie in the posterior part of the brain and send their axon projections forward in a large fasciculated bundle, the MB peduncle, that branches into a number of axon lobes in the anterior brainCitation8 (). In tao mutants, only one of these five sets of axon lobes remains. The axons instead accumulate in a bundle near the MB cell bodies and their dendritic projections in the posterior of the brain. This phenotype has been previously reported for mutants in Rac family small GTPases, and occurs when axons are able to extend, but unable to follow proper paths to the anterior of the brainCitation9 ( and C). In Drosophila Rac family mutants this phenotype occurs in combination with other defects, such as a failure of axons to extend and branch. In tao mutants the axon guidance phenotype is predominant, and no defects in axon extension, cell viability or dendrite morphology are apparent. It therefore appears that tao mutants mainly affect axon pathfinding of MB neurons and do not have the broader range of phenotypes found in Rac family mutants.
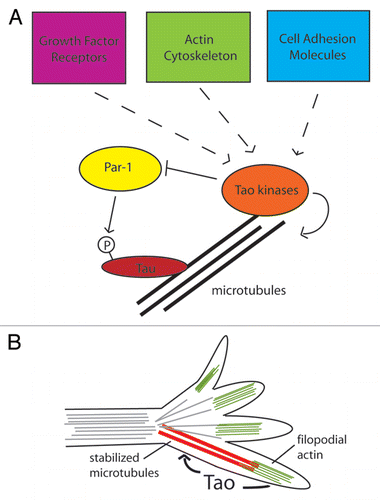
Studies of mammalian Tao1 have shown it to be a regulator of the microtubule cytoskeleton in neurons in vitro. Tao1 can activate the kinase Par-1, which in turn phosphorylates the microtubule-binding protein Tau to enable the extension of neurites in immature neurons.Citation10,Citation11 In flies, mutants in par-1 suppress the MB phenotype of tao mutants, restoring some of the axon lobes. It is therefore very likely that tao affects the microtubule cytoskeleton in Drosophila MB axons as well. However, the genetic relationship between tao and par-1 is reversed in flies, with tao apparently suppressing par-1 activity and Tau phosphorylationCitation12 ().
A growing body of evidence suggests that Tao kinases have a unique role in cytoskeletal control, mediating signaling between the actin and microtubule cytoskeletons. Johne et al. have described an interaction in mammalian cells between Tao1 and the kinase Tesk1, a known regulator of the actin cytoskeleton.Citation13 Tesk1 phosphorylates the actin-binding protein Cofilin, resulting in stabilization of actin filaments. However, Tesk1 also binds to and inhibits Tao1, affecting microtubule dynamics. Tao1 in turn binds the Sprouty-like protein Spred1, which inhibits the activity of Tesk1. This triad of interactions might serve as a point of cross-talk between the microtubule and actin cytoskeletons. Drosophila has homologs of both Tesk1 (center divider or cdi) and Spred1 (Spred), suggesting that this pathway might be conserved, and indeed, over-expression of cdi causes axon pathfinding defects in larval motor neurons.Citation14
Recent work by Baum and colleagues has provided further evidence for tao functioning as a signaling link between the actin cytoskeleton and microtubules.Citation15 Liu et al. show that RNAi knockdown of tao in cultured cells reorganizes the microtubule cytoskeleton in a way that resembles treatments that weaken the cortical actin network. When Drosophila tao levels are knocked down in cultured S2 cells, or when dominant-negative forms of the protein lacking kinase activity are overexpressed, microtubules fail to stop elongating at the cell cortex, resulting in long microtubule-based protrusions. Drosophila tao may thus induce microtubule instability in response to a signal from the actin cytoskeleton at the cell cortex that restrains microtubule growth.Citation15
Taken together, these data suggest a mechanism for Drosophila tao's role in axon guidance (). In advancing axonal growth cones, steering toward or away from directional cues is accomplished by the stabilization microtubules by filopodial actin.Citation16,Citation17 Microtubules extend from the central domain (C-domain) of the growth cone into the actin-supported filopodia of the peripheral domain (P-domain). Signaling from axon guidance cues stabilizes the filopodia, and microtubules extending into the filopodia are in turn stabilized by interaction with filopodial actin. Captured microtubules then provide a track for the extension of the growth cone in a new direction. It is possible that Drosophila tao functions in the process by which the filopodial actin cytoskeleton stabilizes microtubules in response to guidance cues.
Further studies will be needed to untangle the genetic relationships between Drosophila tao and other cytoskeletal regulators. We hypothesize that tao may promote microtubule stability as a negative regulator of Tau phosphorylation. However, the studies using mammalian cells cited above suggest that Tao1 acts to destabilize microtubules. Whether this apparent discrepancy is caused by differences between in vivo (flies) and in vitro (mammalian cells) approaches or by a fundamental difference between flies and mammals remains to be elucidated. In addition, Tao family kinases are likely to transduce signals from a number of sources. For instance, Tao kinases can act downstream of receptor tyrosine kinasesCitation18 and cell adhesion molecules,Citation19 and could therefore be involved in integrating signals from multiple pathways to control axon guidance ().
Figures and Tables
Figure 1 Axon guidance defects in Drosophila tao mutants. (A) The neurons of the mushroom bodies send axon projections from cell bodies in the posterior of the brain. Mushroom body axons form a fasciculated bundle (the peduncle), which branches into three sets of lobes in the anterior of the brain. (B) Mushroom body lobes, visualized with an antibody to Fasciclin II in a wild-type brain and (C) in the brain of a tao mutant fly. In the tao mutant, mushroom body axons fail to follow their proscribed paths to the anterior regions of the brain, instead massing near the cell bodies in the posterior.
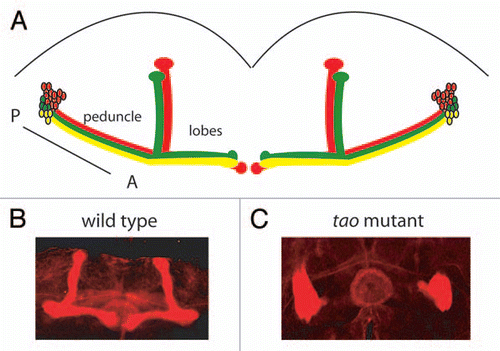
Figure 2 Possible function of Drosophila Tao in cytoskeletal control pathways. (A) Studies of mushroom body development indicate that Drosophila tao acts with par-1 to control phosphorylation of the microtubule binding protein Tau, while in vitro studies suggest that tao may directly stabilize microtubules in response to signals from the actin cytoskeleton. Studies in mammals and Drosophila also suggest that Tao kinases may integrate signals from a number of other upstream sources, such as growth factor receptors and cell-adhesion molecules. (B) Combining these results, we propose that tao might mediate interactions between the actin and microtubule cytosketons in extending growth cones as well, leading to axon guidance defects observed in Drosophila tao mutants. Signals from filopodial actin may act through tao to stabilize and capture extending microtubules.
Addendum to:
References
- Dan I, Watanabe NM, Kusumi A. The Ste20 group kinases as regulators of MAP kinase cascades. Trends Cell Biol 2001; 11:220 - 230
- King I, Tsai LTY, Pflanz R, Voigt A, Lee S, Jäckle H, et al. Drosophila tao Controls Mushroom Body Development and Ethanol-Stimulated Behavior through par-1. J Neurosci 2011; 31:1139 - 1148
- Davis RL. Mushroom bodies and Drosophila learning. Neuron 1993; 11:1 - 14
- Sakai T, Kitamoto T. Differential roles of two major brain structures, mushroom bodies and central complex, for Drosophila male courtship behavior. J Neurobiol 2006; 66:821 - 834
- Hong ST, Bang S, Hyun S, Kang J, Jeong K, Paik D, et al. cAMP signalling in mushroom bodies modulates temperature preference behaviour in Drosophila. Nature 2008; 454:771 - 775
- Joiner WJ, Crocker A, White BH, Sehgal A. Sleep in Drosophila is regulated by adult mushroom bodies. Nature 2006; 441:757 - 760
- Joseph RM, Devineni AV, King IFG, Heberlein U. Oviposition preference for and positional avoidance of acetic acid provide a model for competing behavioral drives in Drosophila. Proc Natl Acad Sci USA 2009; 106:11352 - 11357
- Jefferis GSXE, Marin EC, Watts RJ, Luo L. Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies. Curr Opin Neurobiol 2002; 12:80 - 86
- Ng J, Nardine T, Harms M, Tzu J, Goldstein A, Sun Y, et al. Rac GTPases control axon growth, guidance and branching. Nature 2002; 416:442 - 447
- Timm T, Li XY, Biernat J, Jiao J, Mandelkow E, Vandekerckhove J, Mandelkow EM. MARKK, a Ste20-like kinase, activates the polarity-inducing kinase MARK/PAR-1. EMBO J 2003; 22:5090 - 5101
- Biernat J, Wu YZ, Timm T, Zheng-Fischhofer Q, Mandelkow E, Meijer L, et al. Protein kinase MARK/PAR-1 is required for neurite outgrowth and establishment of neuronal polarity. Mol Biol Cell 2002; 13:4013 - 4028
- Wang JW, Imai Y, Lu B. Activation of PAR-1 kinase and stimulation of tau phosphorylation by diverse signals require the tumor suppressor protein LKB1. J Neurosci 2007; 27:574 - 581
- Johne C, Matenia D, Li Xyu, Timm T, Balusamy K, Mandelkow EM. Spred1 and TESK1—Two new interaction partners of the kinase MARKK/TAO1 that link the microtubule and actin cytoskeleton. Mol Biol Cell 2008; 19:1391 - 1403
- Kraut R, Menon K, Zinn K. A gain-of-function screen for genes controlling motor axon guidance and synaptogenesis in Drosophila. Curr Biol 2001; 11:417 - 430
- Liu T, Rohn JL, Picone R, Kunda P, Baum B. Tao-1 is a negative regulator of microtubule plus-end growth. J Cell Sci 2010; 123:2708 - 2716
- Geraldo S, Gordon-Weeks PR. Cytoskeletal dynamics in growth-cone steering. J Cell Sci 2009; 122:3595 - 3604
- Goode BL, Drubin DG, Barnes G. Functional cooperation between the microtubule and actin cytoskeletons. Curr Opin Cell Biol 2000; 12:63 - 71
- Zhu MY, Wilson R, Leptin M. A screen for genes that influence fibroblast growth factor signal transduction in drosophila. Genetics 2005; 170:767 - 777
- Yasuda S, Tanaka H, Sugiura H, Okamura K, Sakaguchi T, Tran U, et al. Activity-induced protocadherin arcadlin regulates dendritic spine number by triggering N-Cadherin endocytosis via TAO2[beta] and p38 MAP Kinases. Neuron 2007; 56:456 - 471