Abstract
The β2-adrenergic receptor (β2AR) is a prototypical Gs-coupled receptor belonging to the superfamily of seven transmembrane spanning heptahelical receptors (7TMRs, or G protein-coupled receptors [GPCRs])—therapeutically the most diverse and accessible class of cell surface receptors. The classic pathway of β2AR signalling (Fig. 1) is triggered by activation of the heterotrimeric G protein Gs by agonists (catecholamines - noradrenaline and adrenaline). This in turn activates adenylyl cyclase leading to the generation of second messenger signaling molecules (cyclic adenosine monophosphates, cAMP) which subsequently activate protein kinase A (PKA) as well as some ion channels, such as the class C type of L-type calcium channels, CaV1.2. 31 Here in we review how β2AR trafficking and signalling is regulated by the post-translational modification, ubiquitination.1
It is worth mentioning that, the β2 and β1ARs have over the decades been implicated for their physiological importance in maintaining cardiovascular and pulmonary homoeostasis (via the β2AR) and given their significance in modulating adrenergic tone in the heart per se, proved to be the founding basis for the development and characterization of beta-blockers for the failing heart.Citation2,Citation3 In many respects however, the credit to explaining the mechanistic basis of beta-blocker action is unrestrictedly attributed to Lefkowitz and colleagues who contributed towards the discovery and extensive characterization of two novel classes of signaling molecules, viz., the G protein-coupled receptor kinases (GRKs) and the β-arrestins.Citation4,Citation9 Using the β2-subtype of adrenergic receptors as a model system, the past 20 y have significantly highlighted the importance of these two molecules working in concert towards furthering our understanding of homologous desensitization and waning of the receptor signal upon agonist-induced stimulation of 7TMRsCitation4 ().
However, in the past 15 y, β-arrestins have ushered in a paradigmatic shift in the way we look at signal transduction across a cell membrane (). Studies on the angiotensin II Type 1a (AT1aR), the parathyroid hormone type 1 (PTH1R) receptors and other 7TMRs have shown the importance of β-arrestins as signalosome scaffolds in their own right by relaying downstream the signal received from extracellular cues via a mechanism independent of heterotrimeric G protein coupling to a 7TMRCitation5–Citation8 (). In addition to this new concept of biased signaling via β-arrestins and not G proteins at the membrane, we and other have also shown the role of β-arrestins in maintaining the cell surface levels of the β2AR by demonstrating their role in clathrin-dependent endocytosis of 7TMRs.Citation10 In particular, our work has helped demonstrate and attribute a new dimension to ubiquitination, which insofar was generally conceived to mark proteins for degradation by the 26S multi-subunit complex of proteasomal proteases in an ATP-dependent manner and no role whatsoever in regulating 7TMR signaling in terms of cell surface levels as also downstream upon a prolonged tone of agonist-stimulationCitation11 (). As we now see it, ubiquitination is today appreciated as having multi-faceted roles in an array of signaling paradigms including the role of linear polyubiquitin chains in pathophysiological roles of the NFκB pathway in cancer as put forth in the work of Ivan Dikic and Kazuhiro Iwai.Citation11–Citation13
In this mini-review/addenda to our recently published work in The Journal of Biological ChemistryCitation1 we wish to call attention to one such aspect, viz., the role of ubiquitination in mediating downregulation and long-term desensitization of 7TMRs upon agonist-induced stimulation ().
Agonist-stimulated internalization of receptors and trafficking to lysosomes for degradation has been addressed by many studies in sofar. However, the myriad aspects of this regulation lack detailed understanding and one has witnessed the unfolding of novel insights in recent times in this regard. In our recently published work in reference 1, the use of the β2AR as a model system to delineate the motifs involved in ubiquitination of a GPCR and its subsequent targeting to lysosomes appeared justi- fied, for agonist-induced ubiquitination of a mammalian GPCR, regardless of whether the receptor was studied endogenously or in a heterologous expression system, was first reported for the human β2AR.Citation14 However, the hypothesis that ubiquitination as a process per se could regulate cell surface levels of transmembrane proteins was inspired by work on yeast with the yeast peptide transporter, Ste6.Citation15 Until recently, before work on the β2AR and human GPCRs bore prominence, the α-factor pheromone receptor (Ste2p; a GPCR) from Saccharomyces cerevisiae that is hyperphosphorylated and ubiquitinated upon binding to its agonist, the α-factor, served as the essential paradigm to studying GPCR ubiquitination and its effect on the intracellular trafficking pathways of GPCRs.Citation15,Citation16
Ubiquitin is a ubiquitous, small 76 amino acid protein which is attached by a covalent post-translational modification to its target substrate protein at canonical lysine resides, marking it for degradation.Citation17 Typically the extension of the polyubiquitin chain occurs at lysine 48 (K48) or lysine 63 (K63).Citation17 In our previous work, we were able to show that removal of all endogenous lysine residues from the β2AR rendered the receptor incapable of both ubiquitin conjugation and agonist-stimulated degradation, thus confirming the dependence of the receptor degradation on its ubiquitination profile.Citation11 In addition, we demonstrated that ubiquitination of β-arrestins themselves enhanced their propensity for subcellular localization at the membrane enabling formation of tight signalosome complexes with the β2AR leading to receptor endocytosis and concomitant activation of MAP kinase-ERK 1/2.Citation11 In conjunction to this work, we also identified the HECT domain containing E3 ubiquitin ligase, Nedd4 to mediate ubiquitin conjugation to the β2AR thus targeting the receptor to lysosomal compartments for further proteolytic processing.Citation18 However, until recently there has not been a detailed examination and appreciation into highlighting the exact domains in a GPCR where such ubiquitin attachment or conjugation could be ascertained.
Previously, Marchese and Benovic elicited the role of the carboxyl terminus in agonist-promoted ubiquitination and lysosomal sorting of the chemokine receptor, CXCR4.Citation19 However, while mutation of the three carboxyl tail lysines in the SSLKILSKGK motif abrogated CXCR4 ubiquitination and lysosomal sorting, it had no effect on receptor endocytosis.Citation19 In addition a mutation of a single lysine in the vasopressin V2 receptor was also shown to abrogate its ubiquitination and degradation profile.Citation20 Interestingly however, with regard to the β2AR, it was recently demonstrated that lysines in the carboxyl tail of the β2AR were the main sites of receptor ubiquitination but mutation of these lysines did not eliminate receptor ubiquitination, and concomitantly the roles of lysines in other receptor domains of the β2AR were not investigated. Citation21 Hence, in our recent paper in The Journal of Biological Chemistry, we sought to look into this aspect of prolonged agonist-promoted ubiquitination and intracellular trafficking of the β2AR and thereby identify the exact motifs involved in this posttranslation modification of the β2AR combining two approaches including standard biochemistry and confocal microscopy to mass spectrometry-based proteomic analyses.Citation1
In keeping with our previous findings, we observed lysosomal localization of the β2AR upon prolonged agonist-stimulation.Citation11,Citation14,Citation18 However, inhibition of lysosomal proteases led to marked stabilization of the β2AR in lysosomal compartments. In contrast however, when the 26S multi-subunit proteasomal complex was inhibited, the misfolded or immature (not fully glycosylated) β2ARs that escaped the stringent ER quality control machinery were localized in ER resident compartments as highlighted by our observed staining pattern of the β2AR with the ER resident marker for Calreticulin. These observations were validated in experiments conducted in the presence of the protein synthesis inhibitor, cycloheximide (CHX). As mentioned above, we previously demonstrated that mutating all of the endogenous lysines in the β2AR rendered the receptor incapable of ubiquitin conjugation. Hence when the β2AR devoid of all endogenous lysine residues (β2AR-0K) was expressed heterologously, we observed that the β2AR did not localize to lysosomal compartments rather, the bulk of the β2AR pool recycled back to the cell membrane. This experiment clearly elicited the role of β2AR ubiquitination for sorting to lysosomal compartments. In this regard, it is worth noting that as a corollary to this line of thinking, the role of deubiquitination (or the removal of conjugated ubiquitin moieties from target substrate proteins by specialized enzymes termed ubiquitin specific proteases belonging to the family of deubiquitinating enzymes, DUBs) in regulating cell surface levels of GPCRs has been shown for the A2A subtype of adenosine receptors by the ubiquitin specific protease4 (USP4).Citation22 In our own work, we have recently shown that deubiquitination of the β2AR by the USP33, abrogated the localization status of the β2AR in the lysosomes and rather asserted for its accelerated delivery in recycling mode back to the cell membrane.Citation23 Consequently, as expected, the catalytically inefficient mutant of USP33 did not inhibit agonist-induced ubiquitination and lysosomal trafficking of the β2AR—a finding recapitulated with its homolog, viz., USP20.Citation23 These two notable accounts inadvertently showed that ubiquitination/de-ubiquitination act as a specific molecular switch at the level of post-translational modi- fication to maintain the cell surface levels of at least two important, albeit distinct classes of Gs coupled receptors, in response to agonist-stimulation, while at the same time, maintaining a state of homoeostasis in global receptor levels in the cell. Thus, it was but pertinent to work towards delineating the sequence motifs involved in the eventual conjugation of ubiquitin to the β2AR.
From our mutational analysis, we observed that only β2AR-wild type and mutant β2AR with lysines in intracellular loop 3 (L3) and the carboxyl tail (CT) demonstrated ubiquitination, as opposed to the mutants devoid of (β2AR-0K) or harboring individual lysines in the intracellular loops 1 (L1) and 2 (L2). Additionally, it was of particular excitement to learn that ubiquitination within just one of these domains, viz., L3 or CT sufficed akin to the β2AR-wild type to target the receptor to lysosomes after ubiquitination () as determined by radioligand binding experiments. These experiments were successfully validated using a complementary approach (LC-MS/MS analysis) where we sought and indeed identified the exact lysine residues in L3 and CT to mediate the pre-requisite ubiquitin conjugation of the β2AR before it is sorted for lysosomal degradation.
Although a similar approach has previously been employed to reveal novel ubiquitination sites in a variety of signaling molecules including X-linked inhibitors of apoptosis proteins (XIAPs),Citation25 and G proteins themselves,Citation24 to our knowledge, this is the first independent report sought using a mass spectrometrybased proteomic approach towards identifying distinct receptor domains involved in ubiquitination and subsequent intracellular trafficking to lysosomes of a prototypic GPCR upon prolonged agonist-induced stimulation. This approach helped reveal signature peptides with distinct Lys-Gly-Gly branch motifs and a mass shift of 114.0429 Da that allowed the subsequent identification of the candidate lysine residues for ubiquitin conjugation, viz., Lys-263 and Lys-270 in L3 and Lys-348, Lys-372 and Lys-375 in CT. The specificity of the LC-MS/MS approach can well be appreciated from the fact that no ubiquitinated receptor peptides whatsoever were obtained in unstimulated cell samples, hence corroborating our overall experimental findings eliciting ubiquitination as an essential pre-requisite for efficient sorting to lysosomal compartments.
The identification of the sites in the intracellular loop 3 is of prime importance from the consideration of its role in receptor-G protein coupling or recruitment of β-arrestins to GRK-phosphorylated receptors. A decade ago, Gether, Javitch and colleaguesCitation26 had demonstrated the existence of an ionic lock in the β2AR-wild type comprised of a salt bridge between Arg-131 in helix 3 and Glu-268 in helix 6—a fact which was at the time strongly supported by the crystal structure of rhodopsin in the inactivated state.Citation27 Under basal conditions, when the β2AR-wild type is unstimulated and thus in the inactive state, it was hypothesized that the inactive state of the receptor was conceived due to this ionic lock which constrains the movement of the two helices and thus restricts the necessary shift in the conformational equilibrium from the inactive state to the active state of the receptor upon agonist binding. However, by introducing charge neutralizing mutations on Arg-131 and Glu-268 in helix 3 and helix 6, respectively, Gether, Javitch and colleagues not only disrupted the salt bridge interaction between the two residues, but rather signifi- cantly enhanced the basal activity of the β2AR-mutant compared to β2AR-wild type as determined from cAMP accumulation levels in pindolol-treated cell samples transfected with the wild-type or mutant β2ARs thereof in reference 26. Recent crystal structures of non-rhodopsin GPCRs like the β2AR and the β1AR from the groups of Kobilka,Citation28 SchertlerCitation28,Citation29 and TateCitation30 have highlighted the role of helix 3 and helix 6 in maintaining or shifting the conformational equilibrium from the inactive to the active state upon agonist-stimulation and breaking of this ionic lock. Hence, in consonance with the underlying structural framework of receptor activation, we conceive it to be a likelihood that Lys-263 in L3 in close proximity to Glu-268 in helix 6 might correlate with conformational changes and/or receptor activation upon disruption of the ionic lock as an event facilitating agonist-induced ubiquitination of the β2AR. However, it is important to bear in mind that given the lack of structural details regarding the CT that can be ascertained from the various structures of mutant receptors due to crystallographic constraints, the structure-function correlation of ubiquitination at Lys-340, Lys 372 and Lys-375 in the CT is open for debate. But, given that the CT of a GPCR functions as the docking platform for the, recruitment of GRKs and β-arrestins and that GRK phosphorylation precedes β-arrestin recruitment and subsequent trafficking to clathrin-coated pits, the role of these ubiquitinated residues in the CT can in no way be underestimated.
In summary, we have thus validated the previous hypothesis that agonist-induced ubiquitination marks GPCRs for degradation in lysosomal compartments and not proteasomes, for which the latter can be considered largely dispensable. Furthermore, our complementary approach combining proteomics with and standard biochemistry and molecular techniques highlighted the actions of two distinct receptor domains working in concert to direct agonist-induced ubiquitinated β2ARs for lysosomal degradation. It would thus be of general interest for the field to conceive the implication of this finding in terms of specificity or a more generic process while considering the inherent differences in the CT from species and receptor subtypes as elicited in crystal structure determinations.
Figures and Tables
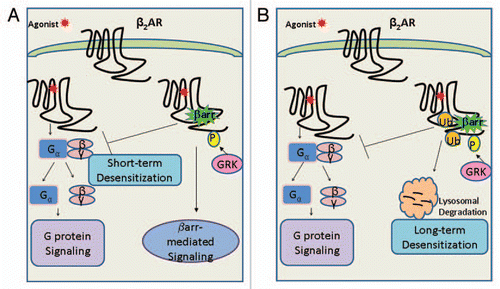
Figure 1 (A) According to the classic GPCR signal ing paradigm, upon agonist binding the β2AR is activated leading to heterotrimeric G protein coupling to the β2AR, dissociation of the Gα from the βγ-subunits and subsequent signaling downstream. Following receptor activation, the agonist-bound β2AR is phosphorylated on its carboxyl tail (CT) by G protein-coupled receptor kinases (GRKs) leading to recruitment of β-arrestins and short-term desensitization of the β2AR. Consequently, β-arrestins also manifest in a second round of extended signaling (independent of G proteins) leading to the prolonged agonist-induced effect as observed in several receptor systems. (B) In addition, the GRK-phosphorylated and β-arrestin scaffolded β2AR is ubiquitinated at lysine residues on two distinct receptor domains: intracellular loop 3 (L3) and the carboxyl tail (CT), which signals the β2AR to lysosomal degradation. The global reduction in cellular receptor levels thus characterizes and provides an explanation for the long-term desensitization of GPCRs upon prolonged agonist-stimulation.
References
- Xiao K, Shenoy SK. β2-adrenergic receptor lysosomal trafficking is regulated by ubiquitination of lysyl residues in two distinct receptor domains. J Biol Chem 2011; 286:12785 - 12795
- Lefkowitz RJ, Rockman HA, Koch WJ. Catecholamines, cardiac beta-adrenergic receptors and heart failure. Circulation 2000; 101:1634 - 1637
- Rockman HA, Koch WJ, Lefkowitz RJ. Seven-transmembrane-spanning receptors and heart function. Nature 2002; 415:206 - 212
- Lefkowitz RJ. Seven transmembrane receptors: a brief personal retrospective. Biochim Biophys Acta 2007; 1768:748 - 755
- Drake MT, Shenoy SK, Lefkowitz RJ. Trafficking of G protein-coupled receptors. Circ Res 2006; 99:570 - 582
- Shenoy SK, Lefkowitz RJ. Angiotensin II-stimulated signaling through G proteins and beta-arrestin. Sci STKE 2005; 311:14
- Gesty-Palmer D, Chen M, Reiter E, Ahn S, Nelson CD, Wang S, et al. Distinct beta-arrestin- and G protein-dependent pathways for parathyroid hormone receptor-stimulated ERK1/2 activation. J Biol Chem 2006; 281:10856 - 10864
- Shenoy SK, Lefkowitz RJ. Seven-transmembrane receptor signaling through beta-arrestin. Sci STKE 2005; 308:10
- Reiter E, Lefkowitz RJ. GRKs and beta-arrestins: roles in receptor silencing, trafficking and signaling. Trends Endocrinol Metab 2006; 17:159 - 165
- Shenoy SK, Lefkowitz RJ. Receptor-specific ubiquitination of beta-arrestin directs assembly and targeting of seven-transmembrane receptor signalosomes. J Biol Chem 2005; 280:15315 - 15324
- Shenoy SK, Barak LS, Xiao K, Ahn S, Berthouze M, Shukla AK, et al. Ubiquitination of beta-arrestin links seven-transmembrane receptor endocytosis and ERK activation. J Biol Chem 2007; 282:29549 - 29562
- Iwai K. Functions of linear ubiquitin chains in the NFκB pathway: linear polyubiquitin in NFκB signaling. Subcell Biochem 2010; 54:100 - 106
- Ikeda F, Deribe YL, Skånland SS, Stieglitz B, Grabbe C, Franz-Wachtel M, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NFκB activity and apoptosis. Nature 2011; 471:637 - 641
- Shenoy SK, McDonald PH, Kohout TA, Lefkowitz RJ. Regulation of receptor fate by ubiquitination of activated beta2-adrenergic receptor and beta-arrestin. Science 2001; 294:1307 - 1313
- Hicke L, Dunn R. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu Rev Cell Dev Biol 2003; 19:141 - 172
- Hicke L, Zanolari B, Riezman H. Cytoplasmic tail phosphorylation of the alpha-factor receptor is required for its ubiquitination and internalization. J Cell Biol 1998; 141:349 - 358
- Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem 1998; 67:425 - 479
- Shenoy SK, Xiao K, Venkataramanan V, Snyder PM, Freedman NJ, Weissman AM. Nedd4 mediates agonist-dependent ubiquitination, lysosomal targeting and degradation of the beta2-adrenergic receptor. J Biol Chem 2008; 283:22166 - 22176
- Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem 2001; 276:45509 - 45512
- Martin NP, Lefkowitz RJ, Shenoy SK. Regulation of V2 vasopressin receptor degradation by agonist-promoted ubiquitination. J Biol Chem 2003; 278:45954 - 45959
- Liang W, Hoang Q, Clark RB, Fishman PH. Accelerated dephosphorylation of the beta2-adrenergic receptor by mutation of the C-terminal lysines: effects on ubiquitination, intracellular trafficking and degradation. Biochemistry 2008; 47:11750 - 11762
- Milojevic T, Reiterer V, Stefan E, Korkhov VM, Dorostkar MM, Ducza E, et al. The ubiquitin-specific protease Usp4 regulates the cell surface level of the A2A receptor. Mol Pharmacol 2006; 69:1083 - 1094
- Berthouze M, Venkataramanan V, Li Y, Shenoy SK. The deubiquitinases USP33 and USP20 coordinate beta2 adrenergic receptor recycling and resensitization. EMBO J 2009; 28:1684 - 1696
- Marotti LA Jr, Newitt R, Wang Y, Aebersold R, Dohlman HG. Direct identification of a G protein ubiquitination site by mass spectrometry. Biochemistry 2002; 41:5067 - 5074
- Shin H, Okada K, Wilkinson JC, Solomon KM, Duckett CS, Reed JC, et al. Identification of ubiquitination sites on the X-linked inhibitor of apoptosis protein. Biochem J 2003; 373:965 - 971
- Ballesteros JA, Jensen AD, Liapakis G, Rasmussen SG, Shi L, Gether U, et al. Activation of the beta2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J Biol Chem 2001; 276:29171 - 29177
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science 2000; 289:739 - 745
- Rosenbaum DM, Cherezov V, Hanson MA, Rasmussen SG, Thian FS, Kobilka TS, et al. GPCR engineering yields high-resolution structural insights into beta2-adrenergic receptor function. Science 2007; 318:1266 - 1273
- Rasmussen SG, Choi HJ, Rosenbaum DM, Kobilka TS, Thian FS, Edwards PC, et al. Crystal structure of the human beta2 adrenergic G-protein-coupled receptor. Nature 2007; 450:383 - 387
- Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AG, et al. The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature 2011; 469:241 - 244
- Davare MA, Avdonin V, Hall DD, Peden EM, Burette A, Weinberg RJ, et al. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 2001; 293:98 - 101