Abstract
Volume homeostasis is a common physiological phenomenon for fluid secreting organs, such as exocrine and endocrine glands. It is a manifestation of a finite intraluminal space and an ever changing demand for secretory fluids. Volume homeostasis addresses issues of fluid secretion, storage and clearance for efficient functioning. Here we discuss the evidence gathered over the past 2-3 decades on serotonin’s role as a feedback inhibitor of secretion in the mammary gland, salivary gland, liver, pancreas, lung, thyroid gland and prostate gland. We propose that serotonin action is a common mechanism of regulating intraductal volume homeostasis.
In any organism, a large fraction of the body is composed of fluid. There are various specialized fluids that perform specific functions e.g., milk provides nourishment to the infant, saliva, pancreatic juice and bile aid in digestion, prostate fluid contributes to semen etc. With the presence of these different specialized fluids arises the issues of their compartmentalization and volume regulation in relation to their demand and physical space available in the organ (volume homeostasis).
The majority of these secretory organs (including exocrine and endocrine glands) have the basic tissue architecture of an arborized ductal network that terminates into numerous spherical lobules, alveoli or acini.Citation1 These ductal and lobuloalveolar structures are lined by epithelial cells that are responsible for production and secretion of the specialized fluids. In addition, the junctions between these epithelial cells (adherence and tight junctions) compartmentalize the ductal and lobuloalveolar structures from the surrounding stroma and thus define the physical space available for fluid secretion. Because of these reasons the ductal and alveolar epithelial cells are paramount for volume homeostasis.
The repertoire of serotonin's (5-Hydroxytryptamine, 5-HT) actions affect virtually all major organ systems including, cardiovascular, pulmonary, gastrointestinal, genitourinary and the central nervous system.Citation2 The serotonin system is highly complex; consisting of rate limiting biosynthetic enzymes tryptophan hydroxylase (TPH), 5-HT reuptake transporter (SERT) which internalizes 5-HT, monoamine oxidases (MaO) which metabolise 5-HT and an extensive network of >20 different receptors that are divided into seven classes (5-HT1–5-HT7) based on pharmacological properties.Citation3
Given the extensive presence and function of 5-HT, here we conduct a highly focused discussion of involvement of 5-HT in volume homeostasis and make the case that this is a common mechanism present across various fluid secreting organs.
Mammary Gland
5-HT action on volume homeostasis has been most extensively studied in the mammary gland. The mammary gland is an exocrine gland that is the most recent acquisition on an evolutionary timescale. It is found in all mammals and its main function is nourishing the infant through milk secretion. Unlike most organs, mammary gland development occurs postnatally in association with pregnancy. The mammary gland secretes milk in a cyclical manner in relation to suckling of the infant and stores milk in between bouts of nursing (milk stasis).Citation4 This necessitates the presence of a reversible feedback inhibitory mechanism to rein in milk secretion in accordance with the volume-space availability of the gland.
A comparative genomic analysis of non-secretory and hyper-secretory mouse mammary glands showed high induction of the 5-HT biosynthetic enzyme, TPH1.Citation5 5-HT biosynthesis was detected during lactation and 5-HT was found in the mammary epithelium and in milk. Interestingly, 5-HT biosynthesis was induced by milk stasis (accumulation) during lactation. 5-HT inhibits milk protein secretion in vivo and in explant cultures.Citation5 Alternatively, 5-HT biosynthesis disruption and 5-HT receptor antagonists significantly enhance secretory features and caused alveolar dilation.Citation5 This led to the conclusion that 5-HT, in an autocrine-paracrine manner, regulates volume homeostasis within the mouse mammary gland. Similar actions of 5-HT in affecting lactation have been observed in humans and bovine.Citation6,Citation7
Human mammary epithelium expresses multiple 5-HT receptors (5-HT1D, 5-HT2B, 5-HT3A and 5-HT7).Citation8,Citation9 Similar observations have been made in rodents and bovine.Citation8–Citation10 Among these, 5-HT7 expression is conserved across species and hence has been studied most extensively.Citation8,Citation9 5-HT7 receptor is localized to the basolateral membrane of mouse and human mammary epithelial cellsCitation8,Citation9 as depicted in the model in . SERT has also been found in human and mouse mammary gland and is localized to the apical membrane of the mammary epithelial cellsCitation6,Citation8,Citation9 ().
A critical transition from a non-secretory mammary epithelium (during pregnancy) to a secretory epithelium (post-partumlactation) is the closure of epithelial tight junctions.Citation11 Tight junction closure compartmentalizes milk secretion and accumulation chamber from the rest of the gland. This is important as persistent leakage of milk into the interstitium is a strong signal for the gland to undergo involution and subsequent tissue remodeling that occurs after weaning.Citation12 Using in vitro models of differentiated human and mouse mammary epithelium,Citation13 5-HT has been shown to breach the tight junction barrierCitation8,Citation14 (). Similar disruptive action of 5-HT on tight junctions has been observed in the bovine mammary glands.Citation15 This action of 5-HT is mediated by its 5-HT7 receptor. Interestingly, 5-HT action on tight junctions was found to be biphasic where 5-HT initially strengthens tight junctions but upon sustained exposure causes disruption of tight junctions.Citation14 This biphasic response is due to an elegant switch in the signaling downstream of the 5-HT7 receptor. The biphasic response to 5-HT is postulated to play an important role during milk stasis which is accompanied by increased intraluminal pressure. Strengthening of tight junctions prevents the leakage of milk into the interstitium in between bouts of nursing (increased pressure). However, upon weaning continued milk stasis breaches the tight junction and induces mammary gland involution.Citation16,Citation17
The impact of 5-HT on milk secretion has been validated in vivo in both bovine and humans via administration of SERT inhibitors (SSRIs) which increase the bioavailability of 5-HT. SSRIs delay the onset of copious milk secretion in primiparous women.Citation6 In bovine, SSRIs suppress milk secretion and accelerate involution (via breach of epithelial tight junctions).Citation15 Hence 5-HT acts as a critical regulator of milk secretion and milk volume in the mammary glands. Other actions of 5-HT in the mammary gland include regulating mammary epithelial turnover via regulating processes such as cell shedding and apoptosis.Citation18 These effects are likely mediated by other receptors present in the mammary epithelium and will not be discussed in detail in this article.
Salivary Glands
The salivary glands are exocrine glands with classic ductal and acinar structure and produce saliva whose main function is to keep food moist while eating. In mammals like humans they also secrete digestive enzymes such as amylases.Citation19 Immunohistochemical analysis of rat submaxillary salivary glands showed the presence of 5-HT in the epithelial cells of excretory ducts and acini.Citation20 The same cells that were positive for 5-HT were also found to contain the Gi coupled (inhibitory) 5-HT1A receptor suggesting an autocrine regulation by 5-HT. In addition, the rat submaxillary glands were also found to contain 5-HT4b and 5-HT7a (Gs coupled stimulatory) receptors indicating a separate autocrine-paracrine role of 5-HT.Citation21 Given the specific (and physiologically opposite) 5-HT receptors, there appears to be more than one autocrine-paracrine action of 5-HT in the rat submaxillary glands.
Analogously, rat parotid salivary glands show opposing effect of 5-HT on secretion of saliva and enzyme amylase.Citation19,Citation22,Citation23 5-HT treatment decreases salivary flow rate but increased amylase secretion. This might be due to action via specific 5-HT receptor subsets which need to be experimentally tested in the parotid gland.
In perfused rat submaxillary glands, 5-HT decreased the saliva flow rate initiated by acetylecholine (Ach).Citation19,Citation22,Citation23 Analysis in the dispersed cell aggregates from the gland showed cAMP accumulation in response to 5-HT suggesting a receptor-mediated action of 5-HT in regulating saliva volume and protein content.Citation19,Citation22,Citation23 Similar cAMP accumulation in response to 5-HT was observed in mouse and opossum submaxillary glands and rat sublingual and parotid glands.
Interestingly, extensive study of insect (blowfly) salivary glands has shown a prominent role of 5-HT (via cAMP pathway) in regulating salivary secretions via modulation electrochemical gradients driving ion transport.Citation24,Citation25 In this system, the 5-HT receptors are localized on the basolateral membrane similar to that seen in the mammary gland as depicted in the model in .Citation26
Liver
The liver is the largest internal organ and the largest gland in the human body. The highly specialized tissues of liver perform various vital functions like detoxification, glycogen storage, protein synthesis, hormone production, decomposition of red blood cells and bile secretion for digestion and emulsification of lipids.Citation27
The biliary tree consists of arborized bile ducts lined by cholangiocytes which are epithelial cells that contribute to bile secretion.Citation28 Cholangiocytes are normally mitotically dormant, but proliferate in response to blockage of bile duct (and subsequent bile accumulation and increased pressure).Citation29
In rats, cholangiocytes synthesize and secrete 5-HT which is increased upon blockage of bile duct (Bile duct ligation-BDL).Citation30 The increased 5-HT has two distinct effects on the biliary tree; it inhibits the proliferation of cholangiocytes and it inhibits the cholangiocyte secretion that contributes towards bile.Citation30 Analogously, Gcoupled (inhibitory) 5-HT1A and 5-HT1B receptors have been found in the basolateral membrane of cholagiocytes and mediate both actions of 5-HT.Citation30 5-HT via 5-HT1A and 5-HT1B inhibits bile and bicarbonate secretion even in presence of the stimulant secretin.
In addition to the autocrine signaling to inhibit bile secretion, 5-HT also stimulates the stromal cells in a paracrine manner to produce TGFβ(1).Citation31 Increased TGFβ(1) counters the autocrine inhibition of bile secretion and proliferation in cholangiocytes by suppressing 5-HT synthesis.
Interestingly, similar to mammary gland, cholestasis induces changes in the tight junction protein distribution.Citation32 This affects the barrier function in an effort to contain bile intraluminally. 5-HT has been shown to affect mammary epithelial tight junction permeabilityCitation8,Citation14 (), however such action of 5-HT in liver has not been explored.
Pancreas
The pancreas consists of two secretory systems, endocrine (insulin secreting β cells) and exocrine (digestive enzyme secreting epithelial cells) system. The 5-HT physiology in the endocrine system will not be discussed here as it is not a part of the volume-homeostatic system.Citation8,Citation14 The exocrine system consists of an extensive ductal network that needs two components operating oppositely and cyclically (digestive and post-digestive periods); a stimulatory input that initiates secretion and a negative feedback input that inhibits secretions when not required. Pancreatic exocrine stimulators are well studied and have been previously reviewed in reference Citation33. In terms of negative feedback, a direct autocrine-paracrine inhibition of exocrine secretion has been documented with 5-HT identified as one of the highly potent local inhibitors.Citation34 Other local factors include arginine, vasopressin, substance P, etc.Citation33
Pancreatic duct cells secrete HCO3- ions to control the basal fluid secretion of pancreatic juice.Citation35 Solitary serotonin producing cells are dispersed across the acinar and ductal epithelium of the exocrine pancreas.Citation35 Isolated guinea pig pancreatic exocrine duct explants have been found to respond to 5-HT.Citation33 Exogenous 5-HT decreased the HCO3- dependent basal fluid secretion of the explants. This inhibitory action of 5-HT was observed even in presence of stimulatory signals like secretin or Acetylcholine (ACh). Interestingly, 5-HT acted specifically through the basolateral side as apical application of 5-HT had no effect. This effect of 5-HT was mediated by 5-HT3 receptor. 5-HT3is a ligand-gated cation channel which by allowing flow of Na+ and Ca2+ ions along the basolateral membrane, dissipates the gradient necessary for intracellular HCO3- accumulation. This intracellular HCO3- accumulation is essential for HCO3- secretion into the lumen. Additionally, increasing intraductal pressure in vivo, decreased fluid secretion and this decrease could be attenuated by 5-HT3 antagonist.Citation33 Hence, the combined evidence documenting 5-HT action on pancreatic exocrine system suggests that the solitary 5-HT cells act as pressure sensors to regulate fluid secretion.
Lungs
In the lungs there are two set of cells that secrete serotonin. Both are collectively called Pulmonary NeuroEndocrine Cells (PNEC). One set of cells is organized into clusters called NeuroEpithelial Bodies (NEB) and are widely studied.Citation33 The second set of cells is arranged in a solitary manner diffused across the airway epithelium and are called NeuroEpithelial Cells (NEC). Physiologically, NECs are quite different than NEBs and only recently began to be investigated.Citation36 5-HT physiology in the NEBs is part of the oxygen sensing apparatus in lungCitation36,Citation37 and hence will not be discussed here. The NECs are widely distributed within the lung epithelium and postulated to be critical in early lung development. These cells extend long dendritic cytoplasmic process along the basement membrane making direct contact with several neighboring epithelial cells as well as epithelial-mesenchymal interaction with subbasement membrane mesenchyme.Citation38,Citation39 Mechanical stretch induces TPH1 and 5-HT release by NECs. Blocking NEC mechanoreceptors attenuates the release of 5-HT.Citation36,Citation37 Mechanical stretch in turn stimulates proliferation of lung epithelial cells and induction of extracellular matrix (ECM) and differentiation of alveolar cells.
Complementing the above study, cultured guinea pig lungs from late gestation fetuses show a 5-HT dose-dependent decrease in the rate of fluid secretion, and activated reabsorption of fluid in the lung.Citation36,Citation37 This phenomenon is critical for the transition of the lungs during childbirth. Furthermore, 5-HT receptor antagonism blocks these effects. Taken altogether, serotonin is induced by stretch, and causes fluid clearance in the late gestation developing lung thus, functioning as a regulator of volume homeostasis in the developing lung.
Thyroid
The thyroid gland is one of the largest endocrine gland and controls energy metabolism, growth and function of many other organ systems of the body. It performs these functions by release of two hormones, triiodothyronin (T3) and thyroxine (T4). Unlike the exocrine glands discussed earlier in this article, the thyroid mainly consists of spherically structures called thyroid follicles made of thyroid epithelial cells. These follicular cells synthesize the thyroid hormones. The centers of these follicles are filled with colloid, which is a proteinacious depot of thyroid hormone precursors. In addition to follicular cells, specific cells nestled between the follicles called parafollicular cells are also present. 5-HT has been localized to both follicular and parafollicular cells.Citation40 Interestingly, 5-HT is also present in the colloid in the follicular lumen and its concentration in the colloid is greater than the follicular cells.Citation41,Citation42 This suggests secretion of 5-HT by the follicular cells. However, localization of SERT only in the follicular cellsCitation41,Citation42 has led to the hypothesis that 5-HT is synthesized by parafollicular cells and acts in a paracrine manner on the follicular cells. Hence, the source of 5-HT synthesis still remains unclear.
Chronic co-treatment of rats with L-tryptophan (a 5-HT precursor that increases 5-HT production) and a SERT inhibitor (increases 5-HT availability) reduced serum levels of thyroid hormone.Citation41–Citation43 Alternatively, chemical inhibition of 5-HT synthesis (DL-p-chlorophenylalanine) or administration of broad spectrum 5-HT receptor antagonist increases serum levels of thyroid hormone.Citation44 These observations indicate that 5-HT regulates thyroid hormone secretion likely in an autocrine-paracrine manner. However, in this case an indirect effect of 5-HT via influence on neural—pituitary axis cannot be ruled out.
Prostate
The prostate gland is an exocrine organ in males that secretes and stores a slightly acidic fluid that contributes towards the final semen. Prostate structure is typical of an exocrine gland with a ductal architecture ending into acini that secrete the fluid. In the prostate, distinct 5-HT producing cells have been found and are referred to as the neuroendocrine (NE) cells.Citation44 The scientific community studying the prostate gland has delved into intensive study of NE cell's involvement in prostate cancer, however there are only a few studies addressing the structure and function of NE cells in normal prostate physiology.
The serotonin producing cells are widely distributed in the prostate epithelium, but are more abundant in the major ducts and irregularly distributed in the acini.Citation45 Analogous to the lung, there are at least two different subpopulations of 5-HT producing cells. One subpopulation (mainly in the peripheral zone) responds to the factors present at birth and at puberty and are suggested to play a role in prostatic growth and differentiation.Citation46,Citation47 These will not be discussed in detail in this article. The second subpopulation mainly resides in prostatic duct system, and are not influenced by the same factors but are postulated to be involved in homeostatic regulation of the secretory process of the gland.Citation45 The morphology of these cells is similar to those found in lung epithelium, where these cells extend dendrite-like processes underneath and between adjacent epithelial cells.Citation45 Based purely on the morphological similarly and physiological comparison with other organs it is postulated that these 5-HT producing cells directly regulate the secretion of adjacent epithelial cells in a paracrine manner.Citation46,Citation47 Along similar lines as that of lung, the prostate 5-HT secreting cells may also be induced to secrete 5-HT by a stretch-activated mechanism. These hypotheses remain to be experimentally validated.
Conclusions
Based on the literature discussed here, it is evident that 5-HT plays a central role in volume homeostasis of various fluid-secreting organs (including endocrine and endocrine). There appears to be a common pattern of 5-HT system in these secretory organs where 5-HT is secreted upon fluid accumulation and/or increased intraluminal pressure (). However, it is important to note that in organs like salivary glands, the wide spread localization of 5-HT may be a manifestation of 5-HT uptake via SERT and only a few subset of cells may actually be synthesizing 5-HT. 5-HT mainly acts as a feedback inhibitor of fluid secretion. Although 5-HT receptor expression in the organs varies widely (), they are mainly found to be located on the basolateral side of the epithelial cells (). Additionally, 5-HT regulation of volume homeostasis in these secretory organs is conserved across multiple species and classes.
All this evidence points to the hypothesis that 5-HT regulation of secretion and volume homeostasis is a very basic and evolutionarily ancient phenomenon that has been adapted and used to suit the needs of various organ systems as they evolved. The abundant repertoire of 5-HT receptors may be a manifestation of this divergent evolution. This hypothesis is supported by the findings that on an evolutionary timeline 5-HT is a very old molecule with function in both plant and animal kingdom.Citation45,Citation48 Also, 5-HT is linked to ion transport and fluid secretion right from establishment of body axis and first epithelial differentiationCitation49 to intestinal fluid and ion transport.Citation50–Citation52
Figures and Tables
Figure 1 Mammary epithelial serotonin system and its mechanism of action. Diagramatic representation of mammary epithelial serotonin system. Mammary epithelial cells synthesize serotonin and secrete it into their surroundings. This serotonin acts in an autocrine-paracrine manner through its receptors. Among these, 5-HT7 receptor has been localized to the baso-lateral side of the mammary epithelial cells. 5-HT through the 5-HT7 receptor generates two signals; a cAMP-PKA signal and a cAMP-p38MAPK signal. Serotonin reuptake transporter (SERT) is present on the apical membrane of the mammary epithelial cells and is involved in recycling and metabolism of mammary serotonin.
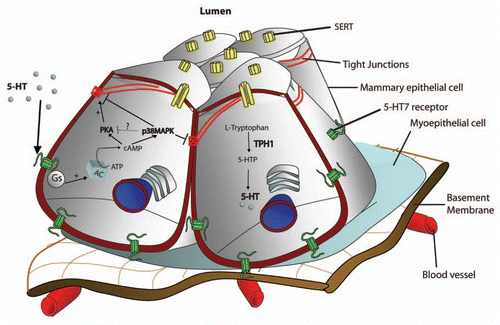
Table 1 Serotonin receptor expression in ductal organs
References
- Affolter M, Bellusci S, Itoh N, Shilo B, Thiery JP, Werb Z. Tube or not tube: remodeling epithelial tissues by branching morphogenesis. Dev Cell 2003; 4:11 - 18
- Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med 2009; 60:355 - 366
- Roth BL. The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics 2006; Totowa NJ Humana Press 618
- McManaman JL, Neville MC. Mammary physiology and milk secretion. Adv Drug Deliv Rev 2003; 55:629 - 641
- Matsuda M, Imaoka T, Vomachka AJ, Gudelsky GA, Hou Z, Mistry M, et al. Serotonin regulates mammary gland development via an autocrine-paracrine loop. Dev Cell 2004; 6:193 - 203
- Marshall AM, Nommsen-Rivers LA, Hernandez LL, Dewey KG, Chantry CJ, Gregerson KA, Horseman ND. Serotonin transport and metabolism in the mammary gland modulates secretory activation and involution. J Clin Endocrinol Metab 2010; 95:837 - 846
- Hernandez LL, Stiening CM, Wheelock JB, Baumgard LH, Parkhurst AM, Collier RJ. Evaluation of serotonin as a feedback inhibitor of lactation in the bovine. J Dairy Sci 2008; 91:1834 - 1844
- Stull MA, Pai V, Vomachka AJ, Marshall AM, Jacob GA, Horseman ND. Mammary gland homeostasis employs serotonergic regulation of epithelial tight junctions. Proc Natl Acad Sci USA 2007; 104:16708 - 16713
- Pai VP, Marshall AM, Hernandez LL, Buckley AR, Horseman ND. Altered serotonin physiology in human breast cancers favors paradoxical growth and cell survival. Breast Cancer Res 2009; 11:81
- Hernandez LL, Limesand SW, Collier JL, Horseman ND, Collier RJ. The bovine mammary gland expresses multiple functional isoforms of serotonin receptors. J Endocrinol 2009; 203:123 - 131
- Nguyen DA, Parlow AF, Neville MC. Hormonal regulation of tight junction closure in the mouse mammary epithelium during the transition from pregnancy to lactation. J Endocrinol 2001; 170:347 - 356
- Stelwagen K, Farr VC, McFadden HA, Prosser CG, Davis SR. Time course of milk accumulation-induced opening of mammary tight junctions and blood clearance of milk components. Am J Physiol 1997; 273:379 - 386
- Marshall AM, Pai VP, Sartor MA, Horseman ND. In vitro multipotent differentiation and barrier function of a human mammary epithelium. Cell Tissue Res 2008; 335:383 - 395
- Pai VP, Horseman ND. Biphasic regulation of mammary epithelial resistance by serotonin through activation of multiple pathways. J Biol Chem 2008; 283:30901 - 30910
- Hernandez LL, Collier JL, Vomachka AJ, Collier RJ, Horseman ND. Suppression of lactation and acceleration of involution in the bovine mammary gland by a selective serotonin reuptake inhibitor. J Endocrinol 2011; 209:45 - 54
- Stein T, Salomonis N, Gusterson BA. Mammary gland involution as a multi-step process. J Mammary Gland Biol Neoplasia 2007; 12:25 - 35
- Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development 1996; 122:181 - 193
- Pai VP, Horseman ND. Multiple cellular responses to serotonin contribute to epithelial homeostasis. PLoS One 2011; 6:17028
- Ostuni MA, Houssay AB, Tumilasci OR. Modulation by thyroid hormones of rat parotid amylase secretion stimulated by 5-hydroxytryptamine. Eur J Oral Sci 2003; 111:492 - 496
- Huang W, Sun L, Lu B, Wang W, Pu R, Chen L, Xia Y. Localization and in situ quantification of 5-hydroxytryptamine and its receptor in rat submaxillary gland. J Mol Histol 2004; 35:47 - 53
- Bourdon DM, Camden JM, Landon LA, Levy FO, Turner JT. Identification of the adenylyl cyclase-activating 5-hydroxytryptamine receptor subtypes expressed in the rat submandibular gland. Br J Pharmacol 2000; 130:104 - 108
- Turner JT, Sullivan DM, Rovira I, Camden JM. A regulatory role in mammalian salivary glands for 5-hydroxytryptamine receptors coupled to increased cyclic AMP production. J Dent Res 1996; 75:935 - 941
- Henriksson R, Sundstrom S, Danielsson A, Hellstrom S. Secretory effects of 5-hydroxytryptamine following neonatal sympathectomy in rat parotid gland. Acta Physiol Scand 1990; 139:597 - 601
- Voss M, Fechner L, Walz B, Baumann O. Calcineurin activity augments cAMP/PKA-dependent activation of V-ATPase in blowfly salivary glands. Am J Physiol Cell Physiol 2010; 298:1047 - 1056
- Zimmermann B, Dames P, Walz B, Baumann O. Distribution and serotonin-induced activation of vacuolar-type H+-ATPase in the salivary glands of the blowfly Calliphora vicina. J Exp Biol 2003; 206:1867 - 1876
- Heslop JP, Berridge MJ. Changes in cyclic AMP and cyclic GMP concentrations during the action of 5-hydroxytryptamine on an insect salivary gland. Biochem J 1980; 192:247 - 255
- Anthea M, Hopkins J, McLaughlin CW, Johnson S, Warner MQ, LaHart D, Wright JD. Human Biology and Health 1993; Englewood Prentice Hall
- Tietz PS, Larusso NF. Cholangiocyte biology. Curr Opin Gastroenterol 2006; 22:279 - 287
- Demorrow S, Francis H, Alpini G. Biogenic amine actions on cholangiocyte function. Exp Biol Med (Maywood) 2007; 232:1005 - 1013
- Marzioni M, Glaser S, Francis H, Marucci L, Benedetti A, Alvaro D, et al. Autocrine/paracrine regulation of the growth of the biliary tree by the neuroendocrine hormone serotonin. Gastroenterology 2005; 128:121 - 137
- Omenetti A, Yang L, Gainetdinov RR, Guy CD, Choi SS, Chen W, et al. Paracrine modulation of cholangiocyte serotonin synthesis orchestrates biliary remodeling in adults. Am J Physiol Gastrointest Liver Physiol 2011; 300:303 - 315
- Maly IP, Landmann L. Bile duct ligation in the rat causes upregulation of ZO-2 and decreased colocalization of claudins with ZO-1 and occludin. Histochem Cell Biol 2008; 129:289 - 299
- Suzuki A, Naruse S, Kitagawa M, Ishiguro H, Yoshikawa T, Ko SB, et al. 5-Hydroxytryptamine strongly inhibits fluid secretion in guinea pig pancreatic duct cells. J Clin Invest 2001; 108:749 - 756
- Garcia M, Hernandez-Lorenzo P, San Roman JI, Calvo JJ. Pancreatic duct secretion: experimental methods, ion transport mechanisms and regulation. J Physiol Biochem 2008; 64:243 - 257
- Hegyi P, Rakonczay Z Jr. The inhibitory pathways of pancreatic ductal bicarbonate secretion. Int J Biochem Cell Biol 2007; 39:25 - 30
- Pan J, Yeger H, Cutz E. Innervation of pulmonary neuroendocrine cells and neuroepithelial bodies in developing rabbit lung. J Histochem Cytochem 2004; 52:379 - 389
- Pan J, Copland I, Post M, Yeger H, Cutz E. Mechanical stretch-induced serotonin release from pulmonary neuroendocrine cells: implications for lung development. Am J Physiol Lung Cell Mol Physiol 2006; 290:185 - 193
- Youngson C, Nurse C, Yeger H, Cutz E. Oxygen sensing in airway chemoreceptors. Nature 1993; 365:153 - 155
- Fu XW, Nurse CA, Wong V, Cutz E. Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbit. J Physiol 2002; 539:503 - 510
- Chua BA, Perks AM. The pulmonary neuroendocrine system and drainage of the fetal lung: effects of serotonin. Gen Comp Endocrinol 1999; 113:374 - 387
- Sciarrillo R, Laforgia V, Virgilio F, Longobardi R, Cavagnuolo A, Varano L. Immunohistochemical localization of NPY, VIP and 5-HT in the thyroid gland of the lizard, Podarcis sicula. Eur J Histochem 2001; 45:377 - 381
- Tamir H, Hsiung SC, Liu KP, Blakely RD, Russo AF, Clark MS, et al. Expression and development of a functional plasmalemmal-5-hydroxytryptamine transporter by thyroid follicular cells. Endocrinology 1996; 137:4475 - 4486
- Spina A, Rea S, De Pasquale V, Mastellone V, Avallone L, Pavone LM. Fate map of serotonin transporter-expressing cells in developing mouse thyroid. Anat Rec 2011; 294:384 - 390
- Masalova OO, Sapronov NS. The role of the serotoninergic system in the regulation of thyroid function in old rats. Bull Exp Biol Med 2009; 148:815 - 818
- Cohen RJ, Glezerson G, Taylor LF, Grundle HA, Naude JH. The neuroendocrine cell population of the human prostate gland. J Urol 1993; 150:365 - 368
- Abrahamsson PA, Wadstrom LB, Alumets J, Falkmer S, Grimelius L. Peptide-hormone- and serotonin-immunoreactive cells in normal and hyperplastic prostate glands. Pathol Res Pract 1986; 181:675 - 683
- Abrahamsson PA, di Sant'Agnese PA. Neuroendocrine cells in the human prostate gland. J Androl 1993; 14:307 - 309
- di Sant'Agnese P. Lechago J, Gould V. Endocrine aspects of the prostate. Bloodworth's Endocrine Pathology 1992; Baltimore Williams & Wilkins
- Park M, Kang K, Park S, Back K. Conversion of 5-hydroxytryptophan into serotonin by tryptophan decarboxylase in plants, Escherichia coli and yeast. Biosci Biotechnol Biochem 2008; 72:2456 - 2458
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol 2005; 15:794 - 803
- Levin M. Is the early left-right axis like a plant, a kidney or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today 2006; 78:191 - 223
- Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyClexpressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Model Mech 2011; 4:67 - 85
- Borman RA, Burleigh DE. Heterogeneity of 5-HT receptors mediating secretion in the human intestine. Ann NY Acad Sci 1997; 812:224 - 225
- Dizeyi N, Bjartell A, Nilsson E, Hansson J, Gadaleanu V, Cross N, Abrahamsson PA. Expression of serotonin receptors and role of serotonin in human prostate cancer tissue and cell lines. Prostate 2004; 59:328 - 336
- Siddiqui EJ, Shabbir M, Mikhailidis DP, Thompson CS, Mumtaz FH. The role of serotonin (5-hydroxytryptamine1A and 1B) receptors in prostate cancer cell proliferation. J Urol 2006; 176:1648 - 1653