Abstract
We recently described the implication of the Bcl-2 related anti-apoptotic Nrz protein during early zebrafish development. Nrz knock-down induces calcium-dependent cytoskeleton remodeling leading to margin constriction and premature embryo lethality. In the YSL, nrz knock-down embryos exhibit some typical features of apoptosis such as mitochondrial transmembrane potential loss and cytochrome c release. However, downstream caspase-3 activation has not been detected so far. Here, we report that the YSL contains fully functional apoptotic machinery that can activate caspase-3 following zBax ectopic expression. Furthermore, we present evidence that caspase-3 activation is actually detectable in nrz knock-down embryos when premature margin constriction is prevented.
In multicellular organisms, programmed cell death is an essential cellular process contributing to embryonic development and morphogenesis. Initial studies conducted on the zebrafish embryo showed that the cell death machinery can be induced at the onset of zygotic gene expression.Citation1,Citation2 During zebrafish development, apoptosis is under the active control of the Bcl-2 family of proteins which primarily regulates the mitochondrial cell death pathway.Citation3 Interestingly, in zebrafish, treatment with chemicals such as nocodazole and camptothecin results in apoptosis in blastomeres located to the enveloping and deep cell layers but not in the yolk syncitial layer (YSL) just beneath.Citation2 YSL is an extramaternal tissue formed by the fusion of margin blastomeres with the yolk cell; it plays critical roles in morphogenesis, nutriments supply and embryo patterning.Citation4 Recently, we showed that the knockdown of the bcl-2 homolog nrz, which is specifically expressed in the YSL, results in premature calcium-dependent actinmyosin ring formation due to enhanced phosphorylation of the myosin light chain (MLC1). Constriction of the actin-myosin ring leads to the detachment of the blastomeres from the yolk cell and premature death of the embryo.Citation5 Together with this lethal phenotype, mitochondrial transmembrane potential (ΔΨm) loss and cytochrome c release were observed in the YSL, whereas no subsequent caspase-3 activation or nuclear fragmentation could be detected, unexpectedly. This raised the idea that upon nrz silencing, separation of the blastomeres from the yolk cell may occur before the caspase cascade could be activated. An alternate possibility was that the YSL might be intrinsically unable to activate caspases and execute apoptosis.
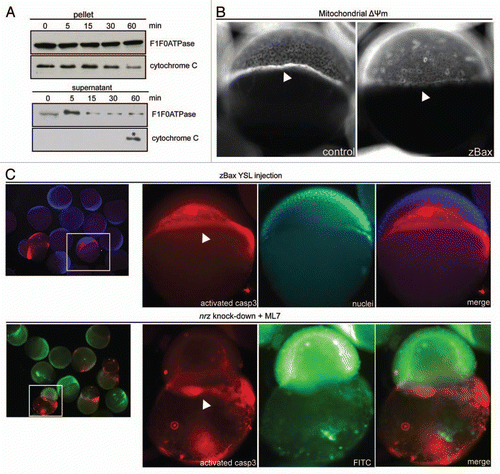
To discriminate between these hypotheses, we first evaluated the capacity of purified YSL mitochondria to release cytochrome c in vitro. For this purpose, YSL mitochondria were incubated with human recombinant tBid protein (20 nM), a known inducer of Baxdependent cytochrome c release. Indeed, tBid treatment of YSL mitochondria induced partial cytochrome c release in the supernatant (), suggesting that in the YSL, endogenous zBax can be activated and induces outer mitochondrial membrane permeability. To further assess the ability of zBax to trigger YSL mitochondria swelling, we injected in vitro synthesized zBax mRNA in the YSL of zebrafish embryos at oblong stage. Interestingly, this resulted in the decrease of ΔΨm mitochondrial potential in the YSL, compared to control embryos, without affecting the blastomeres (). Moreover, this mitochondrial dysfunction appeared to lead to caspase activation. Indeed, immunodetection of active caspase-3 in zebrafish embryos, showed that zBax mRNA injection specifically triggers caspase-3 activation in the YSL (). Together, these results showed that in the YSL the apoptotic machinery is actually fully functional.
These observations prompted us to check if, in nrz knock-down embryos, caspase-3 activation could be detected in nrz-silenced embryos maintained alive by blocking the premature constriction of the actin-myosin ring. For this purpose, zebrafish embryos (1–4 cells stage) were injected with nrz specific antisense morpholino (0.5 mM) and treated with MLCK inhibitor (ML-7; 50 µM) later on. Surprisingly, in such conditions, activated caspase-3 was specifically detected in the YSL in almost 50% of the embryos (44.5%, n = 22) ().
Together, these results indicated that during early zebrafish development, the cell death machinery is mainly controlled by the Nrz protein in the YSL. Indeed, our previous observations demonstrated that during gastrulation, nrz gene expression is restricted to the YSL, contrary to other anti-apoptotic bcl-2 related genes.Citation6 Furthermore, we showed that YSL mitochondria contain significant amounts of Nrz protein whereas other cell death inhibitors, such as Bcl-2 itself, remained undetectable (Bonneau B, et al. unpublished). Thus, Nrz appears to play a dual role in the YSL: (1) in the regulation of calcium trafficking and cytoskeleton remodeling and (2) in the preservation of mitochondrial integrity and apoptosis inhibition.
Why the YSL appears to be resistant to a broad range of apoptotic stimuli remains an open question. Indeed, in zebrafish, the YSL is crucial for early embryonic development, uncontrolled activation of the cell death program during gastrulation being embryonic lethal. Thus it may be speculated that such YSL resistance to death-inducing stimuli is ensured by the anti-apoptotic capacities of Nrz. Further studies will be required to validate this assumption.
Figures and Tables
Figure 1 The zebrafish YSL contains inducible apoptotic machinery. (A) Western blotting detection of cytochrome C from purified YSL mitochondria treated with tBid (20 nM) for increasing time durations (0–60 min). After treatment, mitochondria were pelleted by centrifugation; at 60 min, released cytochrome C due to outer membrane permeabilization was eventually found in the supernatant. The same western blot was probed with anti-F0/F1 ATPase antibody for calibration purpose (top panels). (B) Images of Mitotracker Red-stained embryos injected with control (left panel) or zBax mRNA (right panel). White arrowheads show YSL mitochondria location; zBax-injected embryos exhibit dramatic decrease of the labeling of the YSL mitochondria belt. (C) Immunostaining of activated caspase-3 in zBax-injected embryos (top panels). Embryos were counterstained with nuclear Hoescht33342. Nrz knock-down embryos coinjected with dextran-FITC were treated with ML-7 for 3 h prior staining (bottom panels). Active caspase-3 is mainly found in the YSL (white arrowheads).
Addendum to:
References
- Ikegami R, Zhang J, Rivera-Bennetts AK, Yager TD. Activation of the metaphase checkpoint and an apoptosis programme in the early zebrafish embryo, by treatment with the spindle-destabilising agent nocodazole. Zygote 1997; 5:329 - 350
- Ikegami R, Hunter P, Yager TD. Developmental activation of the capability to undergo checkpoint-induced apoptosis in the early zebrafish embryo. Dev Biol 1999; 209:409 - 433
- Kratz E, Eimon PM, Mukhyala K, Stern H, Zha J, Strasser A, et al. Functional characterization of the Bcl-2 gene family in the zebrafish. Cell Death Differ 2006; 13:1631 - 1640
- Carvalho L, Heisenberg CP. The yolk syncytial layer in early zebrafish development. Trends Cell Biol 20:586 - 592
- Popgeorgiev N, Bonneau B, Ferri KF, Prudent J, Thibaut J, Gillet G. The apoptotic regulator Nrz controls cytoskeletal dynamics via the regulation of Ca(2+) trafficking in the zebrafish blastula. Dev Cell 20:663 - 676
- Arnaud E, Ferri KF, Thibaut J, Haftek-Terreau Z, Aouacheria A, Le Guellec D, et al. The zebrafish bcl-2 homologue Nrz controls development during somitogenesis and gastrulation via apoptosis-dependent and -independent mechanisms. Cell Death Differ 2006; 13:1128 - 1137