Abstract
Synaptic transmission is a major mechanism by which neurons communicate with each other. Basic steps in neurotransmitter release are similar in all synapses. However, many properties of release vary between synapses and reflect specific structural and functional requirements, endowing synapses with specialized functions. Recently, Gelman et al.1 described properties of release and short-term depression at specialized nicotinic synapses in the brainstem of goldfish, Carassius auratus (Linnaeus). These axo-axonic synapses between the Mauthner cell collaterals and their targets, cranial relay neurons (CRNs), exhibit strong short-term depression, even at stimulation frequencies as low as 0.33 Hz. In short, amplitudes of post-synaptic responses, evoked by pre-synaptic trains of action potentials, were depressed with a time course approximated by a sum of two exponential functions. Initially, fast depression reduced the amplitude of EPSP2 (response after the second stimulus), to less than 50% of EPSP1 (response after the first stimulus). This was followed by a slow component of depression that produced an additional 10-30% amplitude reduction over a time-span of tens to hundreds of seconds. Interestingly, depressed EPSPs exhibited longer latencies than that of the ‘undepressed’ EPSP1. Additionally, fast and slow calcium chelators (BAPTA and EGTA), injected pre-synaptically, were equally potent in reducing release. These data are consistent with a previously proposed general mechanism that assumes a change in release probability after the initial release. However, in an alternative interpretation the results could be coherently explained by postulating two releasable pools of vesicles, with high and low release probabilities, and a generally accepted depletion scheme. This latter interpretation will be discussed in this article.
Mauthner (M-) cells are a pair of unique cells found in brainstem of all fish and some amphibians (for a short and funread primer/review, see ref. Citation2). M-cells function as integrators of sensory information. An action potential produced by either of the M-cells generates a fast escape response in a fish by reliably activating downstream connections.Citation3 Thus, M-cells are vital to the survival of the organism and appeared early in the evolution of vertebrates. In fact, modern lampreys and hagfishes, ancient jawless fish whose ancestors, remarkably similar to their descendents, appeared approximately 360 million years ago during the Upper Devonian period,Citation4,Citation5 posses a well-developed M-cell system.Citation6 In modern neurobiology the goldfish M-cell network has been extensively used as a relatively simple model to study, among other things, quantal synaptic transmission and activity-dependent plasticity in the central nervous system.Citation7
Release of synaptic vesicles is a stochastic process. A well-established, quantal theory of synaptic transmission explains the variability in the post-synaptic responses, observed upon stimulation of the pre-synaptic neuron. One of the hallmarks of the theory is that release probability is considered uniform for all releasable vesicles at a single synaptic junction. Constant and uniform release probability is central to a class of depletion models that attribute short-term reduction in synaptic strength to depletion of the neurotransmitter-containing vesicles. However, more recent results from a number of different experiments in various preparations suggest that the release probabilities of synaptic vesicles at single synaptic connections are not uniform (reviewed in ref. Citation8). Two competing mechanistic frameworks attempt to clarify the origin of heterogeneity of release properties among vesicles.Citation8 Heterogeneity may be intrinsic, reflecting differences in exocytotic machinery, calcium sensor and/or vesicle position relative to calcium channels,Citation9 as well as other mechanisms, which would lead to differences in vesicle fusion rate. Alternatively, heterogeneity may not exist a priori, but rather, is induced by previous release. The latter may include calcium adaptation of release machineryCitation10 or calcium-dependent calcium channel inactivation.Citation11 Regardless of the mechanism, attributes of short-term depression that result from the heterogeneity in the properties associated with synaptic vesicles are not clear.Citation12
Mauthner/CRN synapses () have been used to show that a simple depletion model, which assumes a constant probability of release of synaptic vesicles, is inadequate in explaining paired-pulse depression. It has been suggested that at these synapses depression is a consequence of a reduction in release probability (a posteriori heterogeneity mechanism).Citation13
Recently, Gelman et al. described several additional properties of neurotransmitter release and activity dependent short-term plasticity at the M-cell to CRN synapses (). These results are generally consistent with mechanisms that stipulate dynamic reduction in release probability after the initial release, i.e., depression is due to the de-activation of the protein complex involved in exocytosis and is independent of the quantity of neurotransmitter released. Nevertheless, these data could also be interpreted in light of a complex depletion model. In this model, synaptic vesicles possess heterogeneous properties, endowing them with different release probabilities a priori. In a limiting case when the distribution of release probabilities is bimodal, vesicles could be grouped into two pools, a fast releasable pool that is almost entirely depleted after the first stimulus, and a pool consisting of reluctantly releasable vesicles. Findings reported by Gelman et al. illustrating that kinetics of depression are complex, calcium dependent and associated with changes in response latency, are also consistent with the latter mechanism.
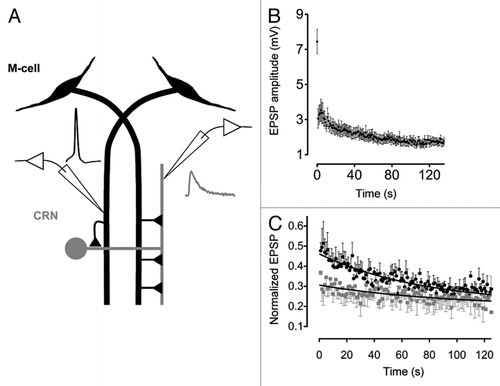
When the M-axon was stimulated at frequencies in the range of 0.33–2.0 Hz, post-synaptic responses quickly depressed. In fact, as illustrated in , EPSP2 is less than 50% that of EPSP1. However, upon subsequent stimulation the rate of depression is decreased, so that EPSP3 (response after the third stimulus) is only slightly smaller than EPSP2. Such change in the kinetics of depression suggests that the release properties of synaptic vesicles that contribute to the first response are different from those that contribute to subsequent responses. This proposition is also supported by the observation that the latency of the first response is shorter by ∼0.1 ms than that of the later responses. Change in the kinetics of depression and response latency, point to multiple changes in the release properties following the first response. These changes may be the result of inherent differences between vesicles from the two pools. For example, one possible scenario could be that very few vesicles are “super-primed” for release and are very quickly exocytosed upon the first stimulation, which leaves behind “reluctant” vesicles with much lower fusion rates. The slow nature of “reluctant” vesicles may be due to slower kinetics of fusion upon binding of calcium ions to the calcium sensor, or due to lower calcium affinity of release proteins, requiring more calcium in the pre-synaptic terminal, or due to the vesicles being positioned further away from calcium channels.Citation14 The latter mechanism is supported by the findings that both BAPTA (fast calcium chelator) and EGTA (slow calcium chelator), injected pre-synaptically, reduce EPSP amplitude to a similar degree (see Fig. 4 and discussion in the original paper; for a review on the positioning of vesicles relative to calcium channels and the consequences, see ref. Citation15 and Citation16).
Data in Gelman et al. indicate that complex kinetics of depression depended on the intracellular levels of calcium ions. This was evident when calcium chelators were pressure-injected into the Mauthner axon. Calcium chelators blocked the second slow component of depression (). Release during this phase, i.e., after the second and subsequent stimuli, was markedly reduced. The amplitude of the EPSP1 was also reduced by the chelators, but only by about 20%. This result could be interpreted in the following way. Lowering intracellular calcium presumably reduced release probability of all of the vesicles in the terminal, effectively rendering “reluctant” vesicles un-releasable and therefore blocking the second, slow component of depression.
In summary, results reported by Gelman et al. suggest that synaptic vesicles in the M-cell terminals contacting CRNs have heterogeneous release properties. First, abrupt reduction in the rate of depression after the second stimulus suggests that the properties of release change after the initial exocytosis. Second, latency increase associated with the change in the kinetics of depression also supports a conclusion that vesicular release after the second and subsequent stimuli occurs with a different time course. Lastly, lowering the intracellular calcium levels, which presumably reduces release probability, has a smaller effect on the first EPSP than on subsequent ones, suggesting that vesicles released after the first stimulus have larger initial release probabilities. As mentioned above, two mechanistic schemes may underlie these observations. Heterogeneity in release probability may be due to inherent differences between vesicles. Alternatively, it may result from a modification in release properties triggered by the initial release.
Figures and Tables
Figure 1 (A) A diagram depicting the M-cell network and recording arrangement. Traces depict M-cell spike (black) generated by injecting depolarizing current and evoked post-synaptic EPSP (gray). (B) EPSP amplitudes during a 1 Hz stimulus train (n = 19 different pairs; mean ± SEM ). (C) Ca2+ buffer's effect on the kinetics of depression. Black circles and gray squares are normalized EPSP amplitudes (EPSP1 is omitted) evoked by pre-synaptic trains of stimulation (1 Hz) before and after injection of 10 mM BAPTA. Black curves are fitted single exponential functions.
Acknowledgments
Original research was conducted in the laboratory of Dr. Donald S. Faber (Albert Einstein College of Medicine). The support was provided by NIH training grant T32NS007439.
Addendum to:
References
- Gelman S, Grove CL, Faber DS. Atypical properties of release and short-term depression at a specialized nicotinic synapse in the Mauthner cell network. J Exp Biol 2011; 214:1560 - 1570
- Sillar KT. Mauthner cells. Curr Biol 2009; 19:353 - 355
- Faber DS, Korn H. Faber DS, Korn H. Electrophysiology of the Mauthner cell: basic properties, synaptic mechanisms and associated networks. Neurobiology of the Mauthner Cell 1978; New York Raven Press 47 - 131
- Janvier P. Palaeontology: modern look for ancient lamprey. Nature 2006; 443:921 - 924
- Gess RW, Coates MI, Rubidge BS. A lamprey from the Devonian period of South Africa. Nature 2006; 443:981 - 984
- Rovainen CM. Physiological and anatomical studies on large neurons of central nervous system of the sea lamprey (Petromyzon marinus). I. Muller and Mauthner cells. J Neurophysiol 1967; 30:1000 - 1023
- Korn H, Faber DS. The Mauthner cell half a century later: a neurobiological model for decision-making?. Neuron 2005; 47:13 - 28
- Schneggenburger R, Sakaba T, Neher E. Vesicle pools and short-term synaptic depression: lessons from a large synapse. Trends Neurosci 2002; 25:206 - 212
- Wadel K, Neher E, Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron 2007; 53:563 - 575
- Hsu SF, Augustine GJ, Jackson MB. Adaptation of Ca2+-triggered exocytosis in presynaptic terminals. Neuron 1996; 17:501 - 512
- Mochida S, Few AP, Scheuer T, Catterall WA. Regulation of presynaptic Ca(V)2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron 2008; 57:210 - 216
- Trommershauser J, Schneggenburger R, Zippelius A, Neher E. Heterogeneous presynaptic release probabilities: functional relevance for short-term plasticity. Biophys J 2003; 84:1563 - 1579
- Waldeck RF, Pereda A, Faber DS. Properties and plasticity of paired-pulse depression at a central synapse. J Neurosci 2000; 20:5312 - 5320
- Moulder KL, Mennerick S. Synaptic vesicles: turning reluctance into action. Neuroscientist 2006; 12:11 - 15
- Augustine GJ. How does calcium trigger neurotransmitter release?. Curr Opin Neurobiol 2001; 11:320 - 326
- Neher E. Vesicle pools and Ca2+ microdomains: new tools for understanding their roles in neurotransmitter release. Neuron 1998; 20:389 - 399