Abstract
The evolution of social life is usually associated with capabilities of individuals to protect group boundaries against foreign individuals. In colonies of ants, the number of reproductive queens is known to influence the accuracy of nestmate discrimination by resident workers. However, the pathway by which this effect is mediated remains unclear. The major hypothesis has long been that workers from multiple-queen colonies commit more discrimination errors against foreigners because their colonies contain a broader diversity of genetically determined cues characterising colony membership. Until recently, this hypothesis has received little attention and poor empirical support. In a recent study, Meunier et al.1 proposed an alternative, albeit not mutually exclusive hypothesis. The presence of one or multiple queens modifies chemical signals on colony members that trigger aggressive or cooperative behaviours during foreign encounters. Here, I detail how this new hypothesis is congruent with previous results and discuss potential limits and evolutionary implications of the two suggested hypotheses.
The defense of group boundaries against intruders is a keystone in the evolution of social life. In colonies of social Hymenoptera (including ants as well as some species of wasps and bees), workers typically carry out this task, often having evolved impressive behaviors and morphological phenotypes to selectively aggress undesired foreigners.Citation2 In insects, the process of nestmate discrimination generally involves the blend of chemical compounds (CC) present on the waxy layer of the cuticle of each individual. These CC are mainly hydrocarbons and convey a diversity of information, e.g., about an individual's genotype, its environment (e.g., nutrition, nest material) or its colony of origin.Citation3,Citation4 To discriminate between nest-mates and foreign individuals, recipient workers generally compare the odours of any encountered intruders to the chemical profile of the recipient colony (= colony - profile), which is obtained through regular exchanges of CC between colony members (e.g., through grooming, trophallaxis or body contacts).Citation3 Mismatches or matches between these two chemical profiles entail aggressive or cooperative behaviors, respectively.
Queen Number and the Diversity of Chemical Signatures
Comparisons across ant species or populations indicate that worker aggressiveness towards conspecific foreigners is larger in single-queen (monogyne) than multiple-queen (polygyne) colonies.Citation5,Citation6 Assuming that CC involved in nestmate discrimination are at least partly genetically determined, the reduced accuracy of nestmate discrimination in polygyne colonies has long been hypothesised to result from the broad diversity of CC within polygyne nests,Citation7 either in quality (i.e., presence/absence of specific CC) or quantity (i.e., same CC at different concentrations). This larger diversity could decrease worker aggressiveness toward foreign individuals due to the possible overlap of CC present across different colonies or simply because it is more difficult for resident workers to compare odors of one individual to the broader colony template. However, the very limited number of empirical studies that tested whether colony-profiles are more complex in polygyne than monogyne colonies provides results that do not support these predictions. In the ant Formica exsecta, monogyne workers are more aggressive towards foreign conspecifics, but intra-nest variability in the relative quantity of alkenes, a family of CC known for their role in nestmate discrimination in this species, is not significantly different between polygyne and monogyne colonies.Citation8 In F. selysi, both the nature and the number of CC collected from workers and eggs were not significantly different between colonies of the two social forms.Citation1 These two studies suggest that factors other than the complexity of colony-profiles could mediate the association between queen number and nest-mate discrimination. However, the limited number of studies indicates the need for further research, either testing correlations between colony social structure and variability of multiple families of CC across field populations, or changes in the complexity of colony-profiles after experimental manipulation of queen number.
Queen Number and the Nature of Chemical Signatures: An Alternative Pathway
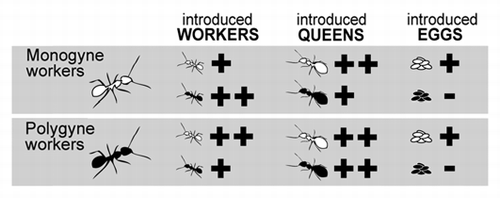
In a recent study, Meunier et al.Citation1 suggested an alternative pathway: queen number influences nestmate discrimination by acting on signals presented by the intruders rather than acting on abilities of the recipient workers. Their hypothesis was built on chemical and behavioral tests in the socially polymorphic ant F. selysi, a species where monogyne and polygyne colonies co-occur in the same population and show no sign of genetic differentiation or reproductive isolation.Citation9,Citation10 The authors showed that recipient workers from the two types of colonies did not differ in their acceptance of foreign eggs and discriminated significantly more against eggs laid by foreign monogyne than polygyne queens, possibly using the chemical signatures specific to each social form.Citation1 Interestingly, previous studies on nestmate discrimination towards workers and queens showed that social origins of introduced adults also triggered different levels of aggressions from recipient workers, a result in line with predictions of the new hypothesis (). In particular, both types of workers were more aggressive towards introduced foreign workers from the alternative rather than identical social form,Citation11 and monogyne workers were significantly more aggressive towards foreign queens of monogyne than polygyne origin.Citation12 The influence of queen number on the chemical signatures of workers has also been reported in two other ant species. In Messor barbatus,Citation13 where the social origin of introduced workers influences their acceptance in monogyne colonies,Citation14 and in the fire ant Solenopsis invicta,Citation15 where nest-mate discrimination depends on the alleles of the gene Gp-9 present in the individuals and in the recipient colony.Citation16
The influence of social origin of intruders on nestmate discrimination raises the questions of its evolution. On the one hand, this effect could be under selection if it provides significant fitness returns for each colony member. Unfortunately, the lack of field data on the frequency and the nature of interactions between monogyne and polygyne individuals make it difficult to predict any fitness benefits entailed by this effect in F. selysi. On the other hand, the influence of the social origin of an intruder on aggressiveness could be a by-product of other intruders' traits that are under selection pressures specific to monogyne or polygyne colonies.Citation17,Citation18 For instance, the costs of nepotistic behaviors of workers over the care of brood from different matrilines in polygyne coloniesCitation19 could select for an egg-signal that prevent workers from discarding eggs according to their maternal origin and by doing so, could trigger the general tolerance of polygyne eggs by foreign workers. Alternatively, the partial overlap of other socially-selected signals of monogyne and polygyne colonies could explain the lower aggression observed between workers from the same versus different social forms.
To conclude, these recent studies revealed that the influence of queen number on nestmate discrimination can be mediated through the signals presented by intruders rather than (or in addition to) the discriminatory ability of recipient workers. The possible existence of such an alternative pathway suggests that general comparisons of nestmate discrimination between monogyne and polygyne species (i.e., species wherein intruders have the same social origin than recipient workers) should be interpreted with caution and may not simply reflect differences in the ability/incentive of resident workers to discriminate against foreigners. Further studies unravelling the proximate and ultimate reasons for selection on colony signals, their direct and indirect influences on social interactions, and their evolution within the social Hymenoptera would provide important insights on the evolution of recognition systems and more generally, on the evolution and maintenance of complex insect societies.
Figures and Tables
Figure 1 The social origin of workers, queens and queen-laid eggs significantly influences the level of aggression they receive from foreign conspecific workers from (white) monogyne and (black) polygyne colonies in the ant Formica selysi. Workers' aggressiveness is (+) high, (++) very high or (−) non-significant. Results are detailed in references Citation1, Citation11, Citation12 and Citation20.
Addendum to:
References
- Meunier J, Delémont O, Lucas C. Recognition in ants: social origin matters. Plos One 2011; 6:19347
- Wilson EO. The Insect Societies 1971; Cambridge MA Harvard University Press
- D'Ettorre G, Lenoir A. Lach L, Parr CL, Abbott K. Nestmate recognition. Ant ecology 2010; Oxford Biology 402
- Blomquist GJ, Bagnères AG. Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology 2010; New York Cambridge University Press
- Bourke AFG, Franks NR. Social Evolution in Ants 1995; Princeton NJ Princeton University Press
- Keller L. Queen Number and Sociality in Insects 1993; Oxford Oxford University Press
- Vander Meer RK, Morel L. Vander Meer RK, Breed M, Winston M, Espelie KE. Nestmate recognition in ants. Pheromone Communication in Social Insects 1998; Boulder Westview 79 - 103
- Martin SJ, Helanterä H, Kiss K, Lee YR, Drijfhout FP. Polygyny reduces rather than increases nestmate discrimination cue diversity in Formica exsecta ants. Insect Soc 2009; 56:375 - 383
- Chapuisat M, Bocherens S, Rosset H. Variable queen number in ant colonies: no impact on queen turnover, inbreeding and population genetic differentiation in the ant Formica selysi. Evolution 2004; 58:1064 - 1072
- Reber A, Meunier J, Chapuisat M. Flexible colony-founding strategies in a socially polymorphic ant. Anim Behav 2010; 78:467 - 472
- Rosset H, Schwander T, Chapuisat M. Nestmate recognition and levels of aggression are not altered by changes in genetic diversity in a socially polymorphic ant. Anim Behav 2007; 74:951 - 956
- Meunier J, Reber A, Chapuisat M. Queen acceptance in a socially polymorphic ant. Anim Behav 2011; 81:163 - 168
- Provost E, Rivière G, Roux M, Bagnères AG, Clément JL. Cuticular hydrocarbons whereby Messor barbarus ant workers putatively discriminate between monogynous and polygynous colonies. Are workers labeled by queens?. J Chem Ecol 1994; 20:2985 - 3003
- Provost E, Cerdan P. Experimental polygyny and colony closure in the ant Messor barbarus (L.) (Hym. Formicidae). Behavior 1990; 115:114 - 126
- Lin YK, Chang HY, Wu WJ, Ho HY, Lin CC. Different cuticular chemical profiles between the monogynous and polygynous forms of the red imported fire ant, Solenopsis invicta (Hymenoptera: Formicidae) in taiwan. Sociobiology 2010; 56:39 - 55
- Gotzek D, Ross KG. Genetic regulation of colony social organization in fire ants: an integrative overview. Quart Rev Biol 2007; 82:201 - 226
- Meunier J, Chapuisat M. The determinants of queen size in a socially polymorphic ant. J Evol Biol 2009; 22:1906 - 1913
- Rosset H, Chapuisat M. Alternative life-histories in a socially polymorphic ant. Evol Ecol 2007; 21:577 - 588
- Holzer B, Kümmerli R, Keller L, Chapuisat M. Sham nepotism as a result of intrinsic differences in brood viability in ants. Proc Roy Soc Lond Ser B 2006; 273:2049 - 2052
- Meunier J, Delaplace L, Chapuisat M. Reproductive conflicts and egg discrimination in a socially polymorphic ant. Behav Ecol Soc 2010; 64:1655 - 1663