Abstract
The conserved Hippo signalling pathway regulates multiple cellular events, including tissue growth, cell fate decision and neuronal homeostasis. While the core Hippo kinase module appears to mediate all the effects of the pathway, various upstream inputs have been identified depending on tissue context. We have recently shown that, in the Drosophila wing imaginal disc, actin-Capping Protein and Hippo pathway activities inhibit F-actin accumulation. In turn, the reduction in F-actin sustains Hpo pathway activity, preventing Yorkie nuclear translocation and the upregulation of proliferation and survival genes. Here, we investigate the role of Capping Protein in growth-unrelated events controlled by the Hippo pathway. We provide evidence that loss of Capping Protein induces degeneration of the adult Drosophila retina through misregulation of the Hippo pathway. We propose a model by which F-actin dynamics might be involved in all processes that require the activity of the core Hippo kinase module.
The conserved Hippo (Hpo) signaling pathway has emerged as a critical regulator of tissue growth both in Drosophila and in mammals. At the center of the pathway are the two Ser/Thr kinases Hpo and Warts (Wts), and their adaptor proteins Salvador (Sav) and dMob as a tumour suppressor (Mats).Citation1–Citation6 This core kinase module represses tissue growth by sequestering the transcriptional co-activator Yorkie (Yki) in the cytosol through phosphorylation and direct binding.Citation7–Citation9 Sequestration of Yki prevents formation of complexes between Yki and DNA-binding transcription co-factors and activation of target genes that regulate cell growth, survival and proliferation.Citation10 In addition to controlling tissue growth, the core kinase module also regulates growth-unrelated events, such as stress-induced apoptosis, cell fate decision and neuronal homeostasis.Citation11–Citation13 Most of these outputs rely on Yki activity, including the control of tissue growth and the maintenance of neuronal homeostasis of the Drosophila adult retina,Citation11 while others are Yki-independent.Citation13
Multiple upstream inputs have also been shown to regulate the core Hpo kinase module at various levels. Among those, the atypical Cadherin Fat was proposed to transduce signals from the atypical Cadherin Dachsous (Ds) and Four-jointed (Fj), while the two Ezrin/Radixin/Moesin (ERM) family members, Expanded (Ex) and Merlin (Mer) are believed to exert their growth suppressive activity by activating the Hpo kinase.Citation14,Citation15 These inputs can act in both a coordinated and independent fashion on Hpo pathway activity depending on the tissue context. In the wing imaginal disc, Fat and Ex are major regulators of the pathway.Citation16,Citation17 In contrast, in the pupal Drosophila eye, Fat and Ex play only minor roles,Citation18 while in the adult retina, Fat, but not Ex, is absolutely critical to prevent neuronal degeneration.Citation11
Despite much progress in understanding the molecular regulation between the core components of this module, key unanswered questions remain, such as how the different inputs are activated and integrated by the core kinase module in various tissues and how this triggers specific developmental outputs.
The Interplay between Hpo Signaling Activity and F-Actin Dynamics
We have recently shown that the actin-Capping protein (CP) heterodimer, composed of an α (Cpa) and β (Cpb) subunits, which regulates actin polymerization, also functions to suppress inappropriate tissue growth by inhibiting Yki activity. Interestingly, Hpo signaling activity, like CP, limits actin filament (F-actin) accumulation at apical sites, independently of Yki. Thus, our findings indicate a novel interplay between Hpo pathway activity and F-actin dynamics, in which regulation of an apical F-actin network by Hpo signaling activity and CP sustains Hpo pathway activity, thereby limiting Yki nuclear import and the activation of proliferation and survival genes.Citation19 Here, we investigate whether CP is also required for growth-unrelated events controlled by the Hpo signaling pathway. We provide evidence that loss of CP induces neuronal degeneration of the adult Drosophila retina through the control of Hpo pathway activity. Based on our work and the increasing number of reports, implicating F-actin in Hpo signaling, we propose that F-actin might be a central player of the pathway, integrating signals from various inputs and mediating tissue-specific outputs.
Capping Protein and Neuronal Degeneration through the HippoSignaling Pathway
Independently of its role in growth control, the Hpo signaling pathway prevents neuronal degeneration of the adult Drosophila retina.Citation11 CP has also been shown to prevent retinal degeneration.Citation20,Citation21 Therefore, CP might have a general requirement in controlling the different outputs of Hpo signaling activity. To test whether loss of CP triggers neuronal degeneration through inhibition of Hpo pathway activity, we carried out genetic interactions between cpa or cpb and components of the hpo signaling pathway. To perform this study, we expressed independent double-stranded RNAs to knock down Cpa (UAS-cpa-IR) or Cpb (UAS-cpb-IR) using the ato348 or GMR-Gal4 drivers, which drive expression in committed G1-arrested cells prior to photoreceptor differentiation in the differentiating retina respectively. While expressing cpb-IR45668 under ato348-Gal4 control did not induce morphological defects (), driving cpa-IRC10 with ato348-Gal4 ( and J) or GMR-Gal4 () or cpb-IR45668 with GMR-Gal4 (data not shown) triggered the appearance of black omatidial clusters, which reveals retinal degeneration. Reducing one copy of hpo strongly enhanced neuronal degeneration of Cpa () or Cpa-depleted photoreceptor cells using either ato348 or GMR-Gal4 (data not shown). While heterozygote mutant animals for hpo, carrying either ato348-() or GMR-Gal4 (data not shown) showed no visible defects of the adult retina. This indicates that CP and hpo genetically interact to maintain neuronal homeostasis.
The neuroprotective function of Hpo signaling required Yki activity.Citation11 Expressing an RNAi construct against Yki with ato348-Gal4 had no visible effect on eye morphogenesis () but suppressed the neurodegenerative phenotype due to Cpa depletion (). We observed a similar suppression with GMR-Gal4 (data not shown). This indicates that Yki activity is part of the signaling cascade, which trigger neuronal degeneration of Cpa-depleted photoreceptors cells.
We then investigated the epistatic relationship between CP and components of the Hpo pathway. Adult eye overexpressing hpo () or expressing both hpo and cpa-IR () under GMR-Gal4 control displayed identical morphological defects. This indicates that Hpo activity overcomes the effect of CP loss. Moreover, ex overexpression, which had no visible effects in the adult eye () suppressed the neuronal degeneration of Cpa-depleted tissues (). Taken together, we conclude that retinal degeneration as a result of CP loss is mediated, at least in part, by inhibiting Hpo pathway activity.
The Actin Cytoskeleton: A Central Role in Hippo Signaling Activity
All outputs of the Hpo signaling pathway seem to be dependent on the activity of the core kinase module, whereas the strength and identity of the upstream inputs depend on tissue context.Citation14,Citation15 The core kinase module is therefore central to the pathway, integrating multiple signals that are translated into tissue-specific responses. We propose that F-actin dynamics may also be a central player of the Hpo signaling pathway (). CP restricts the accessibility of the filament barbed end, inhibiting addition or loss of actin monomers.Citation22 In addition to CP, other actin regulators have also been shown to regulate Hpo signaling activity. Thus, overexpression of a constitutive active form of the actin-nucleator Diaphanous inhibits the pathway activity upstream of Wts but in parallel to Hpo.Citation23 The MST1/2 Hpo orthologs also co-localize with F-actin structures and are activated upon F-actin depolymerization;Citation24 and, Ajuba, which negatively regulates Hpo signaling activity downstream of Hpo and upstream of Wts, belongs to an actin-associated family of LIM-domain-containing proteins.Citation25 In addition, CP appears to be required not only to control Hpo-dependent tissue growth but also Hpo-dependent growth-unrelated events, such as neuronal homeostasis. Thus, CP and consequently F-actin, may mediate all functions of Hpo signaling. Consistent with this, in addition to facilitate Yki phosphorylation by the Hpo kinase cassette in the wing disc tissue,Citation19 CP may also regulate non-phophorylated Yki since Wts-dependent phosphorylation of Yki does not appear to be involved in neuronal homeostasis.Citation11 Moreover, the role of F-actin may be independent of tissue-specific inputs since F-actin seems to be involved in all processes that require the activity of the core kinase module, whereas Ex, which has a critical role in regulating Hpo-dependent tissue growth,Citation16,Citation17 is not involved in neuronal homeostasis.Citation11
In conclusion, the interplay between Hpo signaling activity and F-actin dynamics may be a general requirement in Hpo signaling. Consistent with this, in the wing disc tissue, the core kinase module inhibits F-actin accumulation, prevents excess F-actin of CP-depleted cellsCitation19 and we observed similar epistatic relationships between CP, ex or hpo in the adult retina. Nevertheless, further studies will be required to validate the central role of F-actin in the Hpo pathway.
Materials and Methods
Fly stocks used were UAS-cpa-IRC10,Citation19 UAS-cpa-IR10540R-2; UAS-yki-IR4005R-2 (National Institute of Genetics, NIG); UAS-cpa-IR7009 and UAS-cpb-IR45668 (Vienna Drosophila Research Center, VDRC); UAS-hpoM11.1;Citation2 UAS-ex;Citation4 GMR-Gal4;Citation26 FRT42D, hpo42–48, a gift from D. Pan.Citation1 Crosses with the ato348-Gal4 or GMR-Gal4 drivers were maintained at 25°C or 18°C respectively. Each cross was performed in parallel with the appropriate controls. Adult flies were collected and photographed 24 to 48 h after hatching. To generate ato348-Gal4 transgenic lines that express Gal4 within, and anterior to, the morphogenetic furrow in the eye disc, the minimal 3′-ato eye enhancerCitation27 was cloned in the pChs-Gal4 vector. Transgenic flies were generated by standard methods.
Figures and Tables
Figure 1 Knocking down Capping Protein triggers retinal degeneration through inhibition of Hippo signaling activity. All panels show adult Drosophila retina. The genotypes of the animals are indicated above the panels. ato348 or GMR refers to ato348-Gal4 or GMR-Gal4 driving expression of the indicated transgenes (UAS-cpb45668, UAS-cpa-IRC10, UAS-yki4005R-2, UAS-hpoM11.1 or UAS-ex) and either wild-type for the hpo gene (A) or heterozygote for the hpo42–47 allele (B and C).
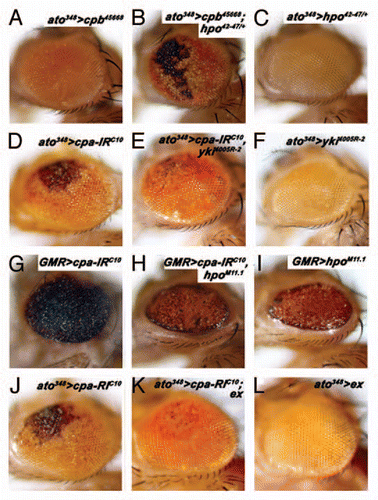
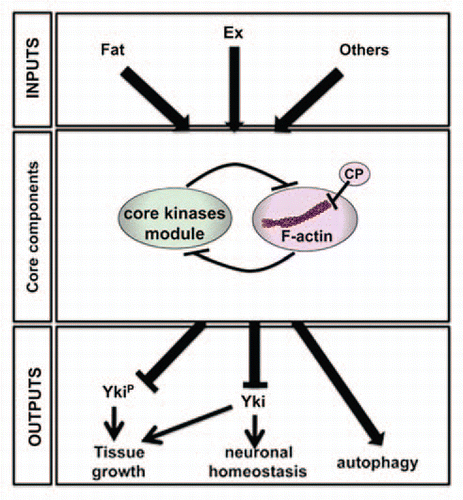
Figure 2 Model for the role of F-actin in Hippo signaling activity. The core kinase module of the Hpo pathway regulates multiple outputs, including tissue growth, neuronal homeostasis and autophagy. Hpo-dependent tissue growth is mediated through inhibition of Yki by a phosphorylation-dependent and independent mechanisms. The neuroprotective effect of Hpo signaling also required Yki inhibition, independent of its phosphorylation by Wts. In contrast, Yki is not involved in Hpo-dependent autophagy. The core kinase complex is regulated by multiple inputs, including Fat and Ex. The interplay between Hpo signaling activity and F-actin dynamics may be a general requirement in Hpo signaling.
Acknowledgments
We thank D.J. Pan, R.G. Fehon, the Bloomington Drosophila Stock Center, the Drosophila Genomics Resource Center, the Vienna Drosophila Research Center (VDRC) and the National Institute of Genetics (NIG) for fly stocks. The manuscript was improved by the critical comments of Beatriz García Fernández and Barbara Jezowska. This work was supported by grants (PTDC/SAU-OBD/73191/2006 and PTDC/BIA-BCM/71674/2006) from Fundação para a Ciência e Tecnologia (FCT). C.B.P. was the recipient of fellowships from FCT (SFRH/BPD/46983/2008), and NEI grant R01EY013167 to F.P.
Addendum to:
References
- Wu S, Huang J, Dong J, Pan D. hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell 2003; 114:445 - 456
- Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell cycle exit in Drosophila. Nat Cell Biol 2003; 5:921 - 927
- Tapon N, Harvey KF, Bell DW, Wahrer DC, Schiripo TA, Haber DA, et al. salvador Promotes both cell cycle exit and apoptosis in Drosophila and is mutated in human cancer cell lines. Cell 2002; 110:467 - 478
- Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol 2003; 5:914 - 920
- Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, et al. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell 2005; 120:675 - 685
- Wei X, Shimizu T, Lai ZC. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J 2007; 26:1772 - 1781
- Oh H, Reddy BV, Irvine KD. Phosphorylationindependent repression of Yorkie in Fat-Hippo signaling. Dev Biol 2009; 335:188 - 197
- Huang J, Wu S, Barrera J, Matthews K, Pan D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005; 122:421 - 434
- Oh H, Irvine KD. In vivo regulation of Yorkie phosphorylation and localization. Development 2008; 135:1081 - 1088
- Reddy BV, Irvine KD. The Fat and Warts signaling pathways: new insights into their regulation, mechanism and conservation. Development 2008; 135:2827 - 2838
- Napoletano F, Occhi S, Calamita P, Volpi V, Blanc E, Charroux B, et al. Polyglutamine Atrophin provokes neurodegeneration in Drosophila by repressing fat. EMBO J 2011; 30:945 - 958
- Mikeladze-Dvali T, Wernet MF, Pistillo D, Mazzoni EO, Teleman AA, Chen YW, et al. The growth regulators warts/lats and melted interact in a bistable loop to specify opposite fates in Drosophila R8 photoreceptors. Cell 2005; 122:775 - 787
- Dutta S, Baehrecke EH. Warts is required for PI3Kregulated growth arrest, autophagy and autophagic cell death in Drosophila. Curr Biol 2008; 18:1466 - 1475
- Grusche FA, Richardson HE, Harvey KF. Upstream regulation of the hippo size control pathway. Curr Biol 2010; 20:574 - 582
- Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development 2010; 138:9 - 22
- Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, Tao C, et al. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signaling to regulate cell proliferation and apoptosis. Nat Cell Biol 2006; 8:27 - 36
- Bryant PJ, Huettner B, Held L Jr, Ryerse J, Szidonya J. Mutations at the fat locus interfere with cell proliferation control and epithelial morphogenesis in Drosophila. Dev Biol 1988; 129:541 - 554
- Milton CC, Zhang X, Albanese NO, Harvey KF. Differential requirement of Salvador-Warts-Hippo pathway members for organ size control in Drosophila melanogaster. Development 137:735 - 743
- Fernandez BG, Gaspar P, Bras-Pereira C, Jezowska B, Rebelo SR, Janody F. Actin-Capping Protein and the Hippo pathway regulate F-actin and tissue growth in Drosophila. Development 2011; 138:2337 - 2346
- Delalle I, Pfleger CM, Buff E, Lueras P, Hariharan IK. Mutations in the Drosophila orthologs of the F-actin capping protein alpha- and beta-subunits cause actin accumulation and subsequent retinal degeneration. Genetics 2005; 171:1757 - 1765
- Johnson RI, Seppa MJ, Cagan RL. The Drosophila CD2AP/CIN85 orthologue Cindr regulates junctions and cytoskeleton dynamics during tissue patterning. J Cell Biol 2008; 180:1191 - 1204
- Cooper JA, Sept D. New insights into mechanism and regulation of actin capping protein. Int Rev Cell Mol Biol 2008; 267:183 - 206
- Sansores-Garcia L, Bossuyt W, Wada KI, Yonemura S, Tao C, Sasaki H, et al. Modulating F-actin organization induces organ growth by affecting the Hippo pathway. EMBO J 2011; 30:2325 - 2335
- Densham RM, O'Neill E, Munro J, Konig I, Anderson K, Kolch W, et al. MST kinases monitor actin cytoskeletal integrity and signal via c-Jun N-terminal kinase stress-activated kinase to regulate p21Waf1/Cip1 stability. Mol Cell Biol 2009; 29:6380 - 6390
- Das Thakur M, Feng Y, Jagannathan R, Seppa MJ, Skeath JB, Longmore GD. Ajuba LIM proteins are negative regulators of the Hippo signaling pathway. Curr Biol 20:657 - 662
- Freeman M. Reiterative use of the EGF receptor triggers differentiation of all cell types in the Drosophila eye. Cell 1996; 87:651 - 660
- Zhang T, Ranade S, Cai CQ, Clouser C, Pignoni F. Direct control of neurogenesis by selector factors in the fly eye: regulation of atonal by Ey and So. Development 2006; 133:4881 - 4889