Abstract
We propose a new in vitro system to study the mechanics of surface area regulation in cells, which takes into an account the spatial confinement of the cell membrane. By coupling a lipid bilayer to the strain-controlled deformation of an elastic sheet, we show that upon straining the supported lipid bilayer expands its surface area by absorbing adherent lipid vesicles and upon compression decreases its area by expelling lipid tubes out of its plane. The processes are reversible and closely resemble in vivo observations on shrinking cells. Our results suggest that the mechanics of the area regulation in cells is controlled primarily by the membrane tension and the effects of the membrane confinement.
Cells communicate with the environment through their membranes, and consequently the membrane surface is a critical parameter for cell function. The membrane defines the cell shape and volume, which vary throughout the life cycle of the cell, e.g., during cell migration,Citation1 cell divisionCitation2 or physiological volume changes in neuronalCitation3 and plant cells.Citation4 Moreover, some specialized tissues, i.e., in the urinary bladder or the lung are subject to cycles of mechanical stretching and compression.Citation5,Citation6 Because the cell membrane is inelastic,Citation7 cells use specific mechanisms to preserve their membrane integrity during area variations. These mechanisms have been discussed in several reviews in reference Citation3, Citation5 and Citation8. For example, cells that are prone to changes in their area maintain an intrinsic membrane reservoir in the form of lipid vesicles. Where there is a demand for area expansion, vesicles are added to the cell membrane (exocytosis) and are retrieved upon compression (endocytosis). The complex morphological transformations of the cell membrane during exo- and endocytosis inevitably require specific protein machinery, but only recently have the material properties of the lipid matrix been recognized as significant.Citation9,Citation10 Experiments with giant vesicles have shown that many of the membrane transformations can be explained by simple energy minimization principles;Citation11 for example, the invaginations in membranes of heterogeneous composition can be driven by their spontaneous curvature,Citation12 membrane fission arises from lipid phase separation,Citation13,Citation14 and increased lipid tension facilitates membrane fusion.Citation9,Citation15
A common feature of the cells in multicellular organisms is that their membranes are in a confined state, i.e., adhered to other membranes or/and an extracellular matrix. Internally, the membrane is pinned to the cytoskeleton. Membrane adhesion is achieved by non-specific physical forces and specific molecular bonds. For example, the adhesion of pure lipid membranes to a substrate or other membranes is a competition between steric repulsion and attraction due to van der Waals and electrostatic forces.Citation16 In addition, cell membranes adhere specifically through various transmembrane and surface proteins, forming desmosomal contacts, tight junctions, receptor-ligand bonds, etc.Citation17 The adhesion forces influence the bending and stretching dynamics of the membrane and may induce local variations in the membrane tension; adhesive interactions also restrict the available volume of the interstitial space. As a result, (i) the mechanics for surface area regulation in spatially confined cells is expected to differ significantly from that in freely suspended cells and (ii) it must be a coordinated process in order to preserve the cell contacts. Nevertheless, the effects of the confinement on the membrane dynamics remain presently unexplored.
In our recent study, we used supported lipid bilayers, coupled to an elastic substrate to address in vitro the effects of the membrane adhesion on the area regulation. By modulating the strain of the substrate, we were able to observe the dynamical response of the strongly adhered bilayer to biaxial expansion and compression. We showed that a lipid membrane subject to lateral stretching expands its area, without compromising its integrity, by incorporating vesicles that are initially adhered to it. The extent to which the membrane can expand depends on the number of adhered vesicles, suggesting their role as a reservoir of lipids. The vesicle absorption is a self-regulated process; it is evenly distributed over the whole membrane area and proceeds continuously throughout the membrane expansion. Our results reproduce observations on real cells, where an increased membrane tension promotes membrane fusion.Citation8 With our experimental setup we visualized details of the tension-induced fusion of a giant vesicle to a planar bilayer (). Briefly, straining the supported bilayer facilitated (i) the adhesion of the giant vesicle,Citation18 and (ii) the hemi-fusion of the contacting membranes. Commonly, the hemifusion precedes the formation of a final fusion pore (, inset).Citation18,Citation19 Instead, we observed an alternative fusion pathway, which had been suggested previously by molecular simulations.Citation20 Pores were formed in the unadhered portion of the vesicle membrane, through which the vesicle content was expelled (iii). The empty vesicle then collapsed (iv) and got absorbed into the expanding supported bilayer (v). This fusion mechanism offers a pathway for area expansion without transporting volume in and out of the cell, which may play an important role during the expansion of cells, such as those confined in tissues.
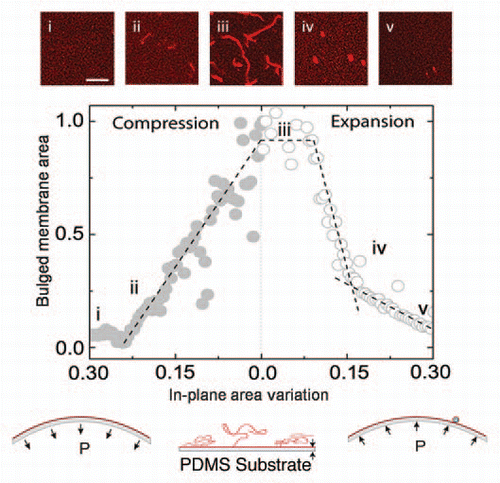
In our experiments with supported lipid bilayers under lateral compression, we show that the confined membrane expels a multitude of lipid tubes to reduce its area in the plane. The tubes nucleate as a result of the destabilization of the bilayer above a certain compression threshold, as has been theoretically suggestedCitation21,Citation22 and elongate throughout the compression (). The lipid tubes are energetically more favorable than spherical buds (1) because the confined membrane tries to minimize the portion of the unadhered membrane and (2) due to insufficient volume to fill the membrane invaginations (i.e., there is only a small interstitial volume and slow water permeability through the membrane). At the cessation of the compression, if left undisturbed the lipid tubes remain stable for a few hours, whereas they retract rapidly and get absorbed by the bilayer if the latter is subjected to stretching (). Under a new cycle of expansion and compression, tubes form and get absorbed reversibly and at roughly the same locations as in the previous cycle.
Our in vitro findings closely reproduce observations on shrinking neurons, T-tubules in skeletal muscles, renal cells, plant cells, etc.Citation3,Citation5,Citation23,Citation24 For example, it has been proposed that the tubular membrane invaginations in these cells serve as a tension/surface area buffer in the demand for rapid surface area variations.Citation23 Because of their reversibility under cycles of area expansion and compression, the membrane tubes are energetically less demanding than the well-known mechanisms of vesicle endo- and exocytosis. In addition, the local reduction of surface area during compression has been proposed as a mechanism for cells to preserve their adhesion contacts during area variations.Citation25
In summary, we have demonstrated that the membrane confinement plays an important role in the surface area regulation of adhered cells. Our experimental setup can be used to study different aspects of membrane adhesion, i.e., inter-membrane distance, covalent pinning, etc., as well as effects of strain rate, membrane composition and osmotic pressure on the morphology of confined membranes.
Figures and Tables
Figure 1 Schematic of the absorption of a giant vesicle (green) into laterally stretched supported bilayer (red). The process passes through the stages (i) adhesion, (ii) hemifusion of the membranes in the contacting area (yellow zones), (iii) pore formation on the unadhered portion of the vesicle membrane and consequent content leakage, (iv) collapse of the vesicle and (vi) final absorption, and as such differs from the commonly observed fusion mechanism through the formation of a fusion pore (inset).
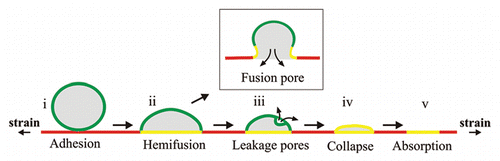
Figure 2 Dynamic transformation of a supported lipid membrane throughout a cycle of area compression and expansion (schematic insets). The area of the out of plane lipid tubes during elongation (compression) and retraction (expansion) is shown as a function of the in-plane membrane area. The insets are confocal images of (i) the bilayer in the initial unstrained conditions, (ii) tube nucleation at a critical compressive threshold, (iii) elongated tubes as a result of the membrane compression, (iv) retraction of tubes to vesicles under membrane expansion and (v) further absorption of the vesicles into the expanding bilayer. Scale bar: 20 microns.
Acknowledgments
We thank M. Arroyo, D.P. Holmes and C. Read for discussions and Princeton University and the Project X Fund for financial support.
Addendum to:
References
- Kay RR, Langridge P, Traynor D, Hoeller O. Surface area regulation: underexplored yet crucial in cell motility. Nat Rev Mol Cell Biol 2008; 9:662
- Boucrot E, Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc Natl Acad Sci USA 2007; 104:7939 - 7944
- Morris E, Homann U. Cell surface area regulation and membrane tension. J Membr Biol 2001; 179:79 - 102
- Shope JC, DeWald DB, Mott KA. Changes in surface area of intact guard cells are correlated with membrane internalization. Plant Physiol 2003; 133:1314 - 1321
- Apodaca G. Modulation of membrane traffic by mechanical stimuli. Am J Physiol Renal Physiol 2002; 282:179 - 190
- Fisher J, Levitan I, Margulies S. Plasma membrane surface increases with tonic stretch of alveolar epithelial cells. Am J Respir Cell Mol Biol 2004; 31:200 - 208
- Evans E, Hochmuth RM. Kleinzeller A, Bronner FEC. Machanochemical properties of membranes. Topics in Membrane and Transport 1978; 10:New York Academic 1 - 64
- Hamill O, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol Rev 2001; 81:685 - 740
- Chernomordik L, Kozlov M. Mechanics of membrane fusion. Nat Struct Mol Biol 2008; 15:675 - 683
- Lenz M, Morlot S, Roux A. Mechanical requirements for membrane fission: common facts from various examples. FEBS Lett 2009; 583:3839 - 3846
- Lipowsky R. The conformation of membranes. Nature 1991; 349:475 - 481
- Fournier JB, Khalifat N, Puff N, Angelova MI. Chemically triggered ejection of membrane tubules controlled by intermonolayer friction. Phys Rev Lett 2009; 102:18102
- Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 2003; 425:821 - 824
- Jülicher F, Lipowsky R. Domain-induced budding of vesicles. Phys Rev Lett 1993; 70:2964 - 2967
- Finkelstein A, Zimmerberg J, Cohen FS. Osmotic swelling of vesicles—Its role in the fusion of vesicles with planar phospholipid-bilayer membranes and its possible role in exocytosis. Ann Rev Physiol 1986; 48:163 - 174
- Israelachvilli JN. Intermolecular and Surface Forces 2011; 3rd edition Waltham Academic Press
- Boal D. Mechanics of the Cell 2002; Cambridge University Press
- Grafmüller A, Shillcock J, Lipowsky R. Pathway of membrane fusion with two tension-dependent energy barriers. Phys Rev Lett 2007; 98:218101
- Haluska C, et al. Time scales of membrane fusion revealed by direct imaging of vesicle fusion with high temporal resolution. Proc Natl Acad Sci USA 2006; 103:15841 - 15846
- Smeijers AF, Markvoort AJ, Pieterse K, Hilbers PAJ. A detailed look at vesicle fusion. J Phys Chem B 2006; 110:13212 - 13219
- Rao M, Sarasij R. Active fusion and fission processes on a fluid membrane. Phys Rev Lett 2001; 87:128101
- Girard P, Juelicher F, Prost J. Fluid membranes exchanging material with external reservoirs. Eur Phys J E 2004; 14:387 - 394
- Krolenko S, Lucy JA. Vacuolation in T-tubules as a model for tubular-vesicular transformations in biomembrane systems. Cell Biol Int 2002; 26:893 - 904
- Li B, et al. Excretion and folding of plasmalemma function to accommodate alterations in guard cell volume during stomatal closure in Vicia faba L. J Exp Botany 2010; 61:3749 - 3758
- Morris CE. Mechanosensitive membrane traffic and an optimal strategy for volume and surface area regulation in CNS neurons. Amer Zool 2001; 41:721 - 727