Abstract
Aging of the nervous system underlies the behavioral and cognitive decline associated with senescence. Understanding the molecular and cellular basis of neuronal aging will therefore contribute to the development of effective treatments for aging and age-associated neurodegenerative disorders. Despite this pressing need, there are surprisingly few animal models that aim at recapitulating neuronal aging in a physiological context. We recently developed a C. elegans model of neuronal aging, and showed that age-dependent neuronal defects are regulated by insulin signaling. We identified electrical activity and epithelial attachment as two critical factors in the maintenance of structural integrity of C. elegans touch receptor neurons. These findings open a new avenue for elucidating the molecular mechanisms that maintain neuronal structures during the course of aging.
Our recent Caenorhabditis elegans model of neuronal aging presents two important features that distinguish it from previous vertebrate models of neurodegenerative diseases.Citation1 First, through the first longitudinal imaging of single neurons across the entire adult lifespan, our model reveals unexpected dynamic features of neuronal aging that are not adequately appreciated in existing animal models of brain aging. Second, our model had identified factors that are specifically required for neuronal integrity without affecting organismal lifespan, indicating that lifespan and cellular aging could be mechanistically uncoupled.
Dynamic Features of Neuronal Aging
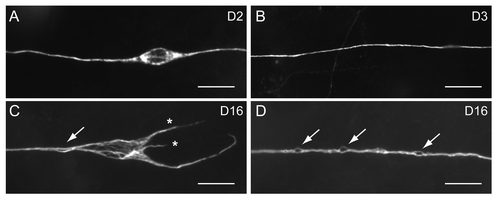
For most metazoan, including C. elegans, aging of the nervous system is not associated with significant neuronal loss.Citation2,Citation3 At the cellular level, the cardinal features of an aging human brain include dystrophic neurites, neurofibrillary tangles and insoluble protein aggregates.Citation2,Citation4,Citation5 How these cellular defects evolve over time is still a mystery. Life-long tracking of individual aging neurons is difficult in lab mammals, however, due to their relatively long lifespan and the extraordinarily huge number of neurons in the mammalian brain. In contrast to previous reports that found no evidence for neuronal aging in C. elegans,Citation3,Citation6 we documented various types of age-dependent defects in C. elegans touch receptor and motor neurons, including misshapen neuronal soma, aberrant neurite formation, and beading or bubble-like lesions in the nerve processes.Citation1 Similar to our findings, Tank et al. had recently reported age-dependent neurite sprouting in C. elegans.Citation7 One striking feature revealed by our longitudinal imaging in touch neurons is the frequent growth and retraction of abnormal neurites. Age-dependent loss of dendritic branches had been found in mammalian neurons.Citation2 By contrast, generation of new neurites in middle and late life of a neuron had never been documented. It is unclear whether these neurites form synaptic connections with other neurons, although they contain acetylated microtubules.Citation1 The emergence of these neurites may represent a failure of the aged neuron to suppress unwanted growth rather than an enhanced ability to generate new structures, judging from their short length, random growth patterns and frequent retraction.Citation1,Citation7 Tank et al. had shown that activity of the Jun kinase pathway is required to suppress these age-dependent neuronal sprouting.Citation7 Since rescued jnk-1 mutant animals overexpressing the C. elegans c-jun N-terminal kinase JNK-1 still show neuronal sprouting comparable to that in age-matched wild type, age-dependent loss of JNK-1 activity cannot be the sole explanation for senescent neuronal sprouting in C. elegans.Citation7 It will be of interest to test whether aberrant neurite outgrowth is regulated by cytoskeletal machinery that also controls developmental axon extension, and what additional mechanisms normally keep these aberrant outgrowth in check.
Another prominent age-dependent defect of C. elegans touch neuron process is beading or bubble-like lesions (), and our longitudinal imaging suggests that some of the minor beading may represent bubble-like lesions in their early phase.Citation1 Axon beading is common in neurons subjected to traumatic, hypoxic or inflammatory insults.Citation8-Citation11 In these conditions, beading may represent cytoskeletal defects or focal accumulation of axoplasmic cargos or organelles due to disrupted axonal transport.Citation8-Citation11 Identity of age-dependent axon beading remains obscure in both human and C. elegans. Interestingly, in some neurons at extremely old age, we observed axon splitting.Citation1 Because multiple bubble-like lesions could be found to coexist on the same axonCitation1 (), a wild speculation is that some of the bubble-like lesions are early focal splitting of the axon. Electron microscopy will be necessary to clarify the nature of these age-dependent axonal defects in C. elegans neurons.
Figure 1. Age-dependent defects of C. elegans touch receptor neurons, revealed by immunostaining of acetylated microtubules. Scale bar, 5 μm. (A) ALM in an animal 2 d of age in adulthood (D2) had a typical spindle-shape soma. (B) The PLM process in a D3 wild type animal. (C) ALM in a D16 animal showed misshapen soma with disorganized microtubule bundles, aberrant neurites (asterisks) and bubble-like lesion of the process (arrow). (D) Multiple bubble-like lesions (arrows) could be found in the PLM process of a D16 animal.
Neuron-Specific Anti-Aging Mechanisms
The stochastic feature of aging describes that in individual animals, the physiological age does not necessarily correlate with its chronological age. Thus, neurons from a mid-age C. elegans may have extensive defects, whereas neurons from an animal at advanced age may appear intact.Citation1,Citation3,Citation6,Citation7,Citation12 On the other hand, individual tissues show great variations in the rate of aging.Citation3,Citation6 However, it is still believed that tissue aging worsens as the chronological age advances, and the regulation of lifespan and cellular aging is tightly linked.Citation3,Citation6,Citation12 We and Tank et al. had identified electrical activities, nerve attachment, JNK and insulin signaling as potentially autonomous factors that contribute to neuronal integrity during aging.Citation1,Citation7 Surprisingly, in both studies, neuronal aging could be uncoupled from organismal aging. Mutations in mec-1 and mec-12, which encode an ECM protein and an α-tubulin, respectively, accelerate neuronal aging without affecting lifespan.Citation1 Similarly, disrupting JNK signaling aggravates age-dependent neurite sprouting but does not decrease lifespan.Citation7 These observations indicate that at the cellular level, aging is regulated in a tissue-specific manner, and is not necessarily linked to lifespan regulation.
Electrical activity had been shown to be essential for adult Drosophila olfactory neurons to survive and for newly generated hippocampal neurons to integrate into the adult neural circuits.Citation13,Citation14 Our study extends the roles of electrical activity in the maintenance of adult nervous system, and raises several issues that await exploration in the near future. First, does physiological membrane activity constantly activate signaling pathways that promote neuronal integrity, or does it act to suppress cellular machinery that dismantles the neurons? These two possibilities are not mutually exclusive and may cooperate to achieve optimal neuronal maintenance against aging.
Second, what is the signaling pathway that translates electrical activity on the plasma membrane into transcriptional and posttranslational mechanisms for neuronal maintenance? Calcium emerges as a promising target, in that its wide spectrum of intracellular dynamics makes it one of the most versatile second messengers in cellular physiology. Previous studies indicate that aged hippocampal neurons show more pronounced increase in intracellular calcium level under stimulation, compared with young neurons.Citation15 Deranged regulation of intracellular calcium may underlie some of the functional decline seen in the aged neurons.Citation16 In larval C. elegans, calcium had been shown to mediate necrotic neuronal death induced by excessive sodium channel activity.Citation17 On the other hand, regeneration of touch neuron processes after laser axotomy requires calcium.Citation18 Whether calcium promotes regenerative behaviors of injured neurons or causes the neuron to die may depend on its temporal dynamics. Rapid, massive calcium increase in the cytosol may activate proteolytic enzymes that dismantle the neuron, whereas persistent, low-grade calcium flow could activate cellular pathways that contribute to axon repair or neuronal integrity in the long run. One of the future challenges will be to monitor neuronal calcium dynamics in an extended temporal dimension that is relevant to aging.
In conclusion, the C. elegans model of neuronal aging reveals novel age-dependent defects that are highly dynamic, and had identified several factors specifically required for the maintenance of neuronal integrity during aging. It is important to verify these observations in other organisms such as Drosophila, mouse or human. A future goal will be to unravel the genetic control of age-dependent neuronal defects through forward and reverse genetics approaches.
References
- Pan CL, Peng CY, Chen CH, McIntire SL. Genetic analysis of age-dependent defects of the Caenorhabditis elegans touch receptor neurons. Proc Natl Acad Sci USA 2011; 108:9274 - 9; http://dx.doi.org/10.1073/pnas.1011711108; PMID: 21571636
- Yankner BA, Lu T, Loerch P. The aging brain. Annu Rev Pathol 2008; 3:41 - 66; http://dx.doi.org/10.1146/annurev.pathmechdis.2.010506.092044; PMID: 18039130
- Herndon LA, Schmeissner PJ, Dudaronek JM, Brown PA, Listner KM, Sakano Y, et al. Stochastic and genetic factors influence tissue-specific decline in ageing C. elegans.. Nature 2002; 419:808 - 14; http://dx.doi.org/10.1038/nature01135; PMID: 12397350
- Wiśniewski HM, Ghetti B, Terry RD. Neuritic (senile) plaques and filamentous changes in aged rhesus monkeys. J Neuropathol Exp Neurol 1973; 32:566 - 84; http://dx.doi.org/10.1097/00005072-197310000-00007; PMID: 4202280
- Wiśniewski HM, Terry RD. Morphology of the aging brain, human and animal. Prog Brain Res 1973; 40:167 - 86; http://dx.doi.org/10.1016/S0079-6123(08)60686-X; PMID: 4371053
- Haithcock E, Dayani Y, Neufeld E, Zahand AJ, Feinstein N, Mattout A, et al. Age-related changes of nuclear architecture in Caenorhabditis elegans.. Proc Natl Acad Sci USA 2005; 102:16690 - 5; http://dx.doi.org/10.1073/pnas.0506955102; PMID: 16269543
- Tank EM, Rodgers KE, Kenyon C. Spontaneous Age-related neurite branching in Caenorhabditis elegans.. J Neurosci 2011; 31:9279 - 88; http://dx.doi.org/10.1523/JNEUROSCI.6606-10.2011; PMID: 21697377
- Kim JY, Shen S, Dietz K, He Y, Howell O, Reynolds R, et al. HDAC1 nuclear export induced by pathological conditions is essential for the onset of axonal damage. Nat Neurosci 2010; 13:180 - 9; http://dx.doi.org/10.1038/nn.2471; PMID: 20037577
- Ochs S, Pourmand R, Jersild RA Jr., Friedman RN. The origin and nature of beading: a reversible transformation of the shape of nerve fibers. Prog Neurobiol 1997; 52:391 - 426; http://dx.doi.org/10.1016/S0301-0082(97)00022-1; PMID: 9304699
- Nakayama Y, Aoki Y, Niitsu H. Studies on the mechanisms responsible for the formation of focal swellings on neuronal processes using a novel in vitro model of axonal injury. J Neurotrauma 2001; 18:545 - 54; http://dx.doi.org/10.1089/089771501300227341; PMID: 11393257
- Fayaz I, Tator CH. Modeling axonal injury in vitro: injury and regeneration following acute neuritic trauma. J Neurosci Methods 2000; 102:69 - 79; http://dx.doi.org/10.1016/S0165-0270(00)00282-X; PMID: 11000413
- Garigan D, Hsu AL, Fraser AG, Kamath RS, Ahringer J, Kenyon C. Genetic analysis of tissue aging in Caenorhabditis elegans: a role for heat-shock factor and bacterial proliferation. Genetics 2002; 161:1101 - 12; PMID: 12136014
- Chiang A, Priya R, Ramaswami M, Vijayraghavan K, Rodrigues V. Neuronal activity and Wnt signaling act through Gsk3-beta to regulate axonal integrity in mature Drosophila olfactory sensory neurons. Development 2009; 136:1273 - 82; http://dx.doi.org/10.1242/dev.031377; PMID: 19304886
- Lin CW, Sim S, Ainsworth A, Okada M, Kelsch W, Lois C. Genetically increased cell-intrinsic excitability enhances neuronal integration into adult brain circuits. Neuron 2010; 65:32 - 9; http://dx.doi.org/10.1016/j.neuron.2009.12.001; PMID: 20152111
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci 2010; 33:153 - 61; http://dx.doi.org/10.1016/j.tins.2009.12.003; PMID: 20071039
- Toescu EC, Vreugdenhil M. Calcium and normal brain ageing. Cell Calcium 2010; 47:158 - 64; http://dx.doi.org/10.1016/j.ceca.2009.11.013; PMID: 20045187
- Xu K, Tavernarakis N, Driscoll M. Necrotic cell death in C. elegans requires the function of calreticulin and regulators of Ca2+ release from the endoplasmic reticulum. Neuron 2001; 31:957 - 71; http://dx.doi.org/10.1016/S0896-6273(01)00432-9; PMID: 11580896
- Ghosh-Roy A, Wu Z, Goncharov A, Jin Y, Chisholm AD. Calcium and cyclic AMP promote axonal regeneration in Caenorhabditis elegans and require DLK-1 kinase. J Neurosci 2010; 30:3175 - 83; http://dx.doi.org/10.1523/JNEUROSCI.5464-09.2010; PMID: 20203177