Abstract
Membrane Type-1 Matrix Metalloproteinase (MT1-MMP, MMP-14) is regarded as the prototype of a membrane- tethered protease. It drives fundamental biological processes ranging from embryogenesis to cancer metastasis. The proteolytic cleavage of proteins by MT1-MMP can rapidly alter the biophysical properties of a cell’s microenvironment. Cell’s must thus be able to sense and react to these alterations and transduce these effectively in biochemical signals and cell responses. Although many cells react as acutely to such physical stimuli as they do to chemical ones, the regulatory effects of these have been less extensively explored. In order to investigate a possible interdependency of proteolytic matrix cleavage by MT1-MMP and the generation and sensing of force by cells, a model system was established which exploits the properties of a matrix array of parallel collagen-I fibers. The resulting an-isotropy of the matrix with high tensile strength along the fibers and high mobility perpendicular to it allows the convenient detection of bundling and cleavage of the collagen fibers, as well as spreading and durotaxis of the cells. In summary, we have demonstrated that cell adhesion, force generation, and force sensing are vital for the regulation of MT1-MMP for efficient cleavage of collagen-I.
The function of proteases constitutes a common necessity in any tissue remodeling process, ranging from embryogenesis to cancer.Citation1-Citation4 As such, it was recognized early on that the metastatic potential of cancer cells relies on the ability to degrade the extracellular matrix (ECM) of basement membranes.Citation5 Nevertheless the complex action of proteases has to be regulated beyond a simple “path-clearing-process”Citation6. The ECM can be regarded not only as a physical obstacle, but also as a scaffold which provides sufficient resistance to allow cells the development of traction for migration. Cancer cells would benefit from proteolysis as an instrument to modulate (1) their attachment to and detachment from the ECM, (2) the local biomechanical properties of the ECM, and (3) the overall structure of their microenvironment.Citation7 The ability to regulate matrix proteolysis and sense the micromechanics of their environment could be regarded to be essential in this context.
In our recent work we hypothesized a putative interdependency of cellular matrix adhesion, force exertion on the matrix and proteolysis for ECM remodeling.Citation8 An anisotropic matrix of fluorescently labeled, parallel collagen-I fibers proved to be versatile platform to visualize these interdependencies by atomic force microscopy (AFM) or fluorescence microscopy. Labeling of the collagen matrix with fluorophores simplifies the visualization of the cellular response.Citation9-Citation12
A human melanoma cell line (MV3) transfected with MT1-MMP or an empty control vector (MOCK) served as a model.Citation8 These cells have been shown to express α2β1 integrin for collagen-I adhesion and have been used for cell migration studies previously.Citation13,Citation14 By seeding MV3 cells that stably express MT1-MMP onto the parallel collagen matrix we could assess their responses to a number of conditions which influences their ability to adhere to, or generate forces (e.g., pull) on the collagen fibers. The interaction of the MV3 cells with the parallel collagen is easy to visualize, displacement or cleavage of the fibers can be evaluated from the images in a “binary” –yes or no –fashion.
After exposing the collagen lattice for one hour to the MV3 cells, it become apparent that matrix anisotropy was essential for the detection of proteolysis and that expression of MT1-MMP causes the formation of “floppy” collagen fibers (). Despite the expression of various soluble proteases by the MV3 cells, the proteolysis depended on the expression of the membrane bound MT1-MMP and remained a very local phenomenon.Citation15 This is in line with previous functional observations by us, where proteolysis was found to be a highly regulated and local phenomenon.Citation1,Citation16-Citation18
Figure 1. Representative confocal images and schematic representations of melanoma cells (MV3-clone). The cells express either MT1-MMP (+MT1-MMP) or an empty control vector (MOCK) and were exposed to isotropic or an-isotropic matrices of collagen-I for one hour. An MV3 cell expressing (a) an empty control vector or (b) MT1-MMP on an isotropic collagen matrix show that features of the interaction of the cells with the collagen are difficult to detect. (c) The parallel alignment of collagen fibers causes matrix an-isotropy, with high tensile strength along the fibers and high mobility and pliability of the fibers perpendicular to it (arrows). MV3-cells align along the fibers, but show no features of matrix cleavage under control conditions. (d) Expression of MT1-MMP in cells that are exposed to an an-isotropic matrix induces visible matrix defects. However, pharmacological interference with cell adhesion, force generation or force sensing abolishes the cell’s ability to cleave the fibers. (e) The addition of cytochalasin D breaks down the actin network. The cells are still able to attach to the collagen, but unable to generate any force. (f) Blocking α2β1-integrins with antibodies inhibits the cell’s ability to attach to th collagen. The cells squeeze themselves between the collagen layer and the solid support, a process also known as durotaxis. (g) Blebbistatin inhibits non-muscular myosin. Even low concentrations (1 µM) are enough to interfere with the cell’s ability to cleave and bundle the collagen fibers.(scale bar 10 µm).
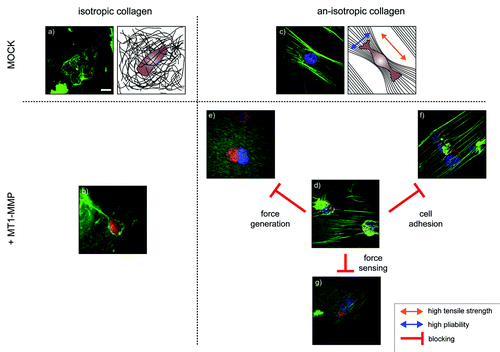
Since MV3 cells rely on α2β1 integrins to adhere to collagen-I, these were blocked by corresponding antibodies. Strikingly, after seeding the cells onto the parallel collagen matrix in the presence of these antibodies collagen fiber cleavage decreased by more than 90% (). Another striking aspect was the promoted durotaxis, a phenomenon marked by the migration of cells toward a stiffer environment, observable here by cells residing between the collagen matrix and the underlying support. It could be concluded that integrin-based adhesion either impairs durotaxis or stiffness sensing is dysregulated in the absence of functional adhesion. However, the function of collagen binding integrins is in this context vital for the efficacy of matrix cleavage by MT1-MMP. Whether this is linked to cell adhesion and force generation as such, or compartmentalization of MT1-MMP into membrane microdomains by integrins remains to be elucidated.Citation19,Citation20 Independent from this consideration and the relevance of integrins for the generation of cell traction given, we hypothesized actually that force generation or sensing would nonetheless be vital in either of these processes.
Based on the aforementioned results, the actin cytoskeletal and myosin II functionality were blocked individually. In essence, the dependence of collagen-I remodeling and fiber cleavage on the cells ability to either (1) exert force onto the substrate or (2) the ability to sense it were probed individually. It was hypothesized recently that acto-myosin contractile units act as mechanosensors that adapt mechanical power to the stiffness of the cell substrate.Citation21 In short, breaking down the actin cytoskeleton with cytochalasin D () or inhibiting myosin II with blebbistatin () led to the complete absence of cleaved collagen fibers one hour after seeding the cells onto the parallel collagen. Interestingly, neither of the substances had an acute effect on the presence of MT1-MMP on the cells’ surface.Citation22
In summary, we have demonstrated a stringent dependency of extracellular proteolysis from force generation, force sensing, and cellular adhesion. The mechanisms for the integration of physical and biochemical processes on the cellular level remain to be elucidated. It can, however, be concluded that methods which enable the quantification of basic biophysical parameters such as cell and matrix stiffness on a subcellular level will be of major significance.Citation7
| Abbreviations: | ||
| AFM | = | atomic force microscopy |
| ECM | = | extracellular matrix |
| MT1-MMP | = | Membrane Type-1 Matrix Metalloproteinase |
| MV3 | = | MV3-clone of melanoma cells |
References
- Ludwig T. Local proteolytic activity in tumor cell invasion and metastasis. Bioessays 2005; 27:1181 - 91; http://dx.doi.org/10.1002/bies.20306; PMID: 16237672
- Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 2001; 17:463 - 516; http://dx.doi.org/10.1146/annurev.cellbio.17.1.463; PMID: 11687497
- Page-McCaw A, Ewald AJ, Werb Z. Matrix metalloproteinases and the regulation of tissue remodelling. Nat Rev Mol Cell Biol 2007; 8:221 - 33; http://dx.doi.org/10.1038/nrm2125; PMID: 17318226
- Gross J, Lapiere CM. Collagenolytic activity in amphibian tissues: a tissue culture assay. Proc Natl Acad Sci U S A 1962; 48:1014 - 22; http://dx.doi.org/10.1073/pnas.48.6.1014; PMID: 13902219
- Liotta LA, Tryggvason K, Garbisa S, Hart I, Foltz CM, Shafie S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980; 284:67 - 8; http://dx.doi.org/10.1038/284067a0; PMID: 6243750
- Stamenkovic I. Extracellular matrix remodelling: the role of matrix metalloproteinases. J Pathol 2003; 200:448 - 64; http://dx.doi.org/10.1002/path.1400; PMID: 12845612
- Ludwig T, Kirmse R, Poole K, Schwarz US. Probing cellular microenvironments and tissue remodeling by atomic force microscopy. Pflugers Arch 2008; 456:29 - 49; http://dx.doi.org/10.1007/s00424-007-0398-9; PMID: 18058123
- Kirmse R, Otto H, Ludwig T. Interdependency of cell adhesion, force generation and extracellular proteolysis in matrix remodeling. J Cell Sci 2011;; 124:1857 - 66; http://dx.doi.org/10.1242/jcs.079343; PMID: 21558415
- Jiang F, Khairy K, Poole K, Howard J, Müller DJ. Creating nanoscopic collagen matrices using atomic force microscopy. Microsc Res Tech 2004; 64:435 - 40; http://dx.doi.org/10.1002/jemt.20101; PMID: 15549696
- Friedrichs J, Taubenberger A, Franz CM, Muller DJ. Cellular remodelling of individual collagen fibrils visualized by time-lapse AFM. J Mol Biol 2007; 372:594 - 607; http://dx.doi.org/10.1016/j.jmb.2007.06.078; PMID: 17686490
- Poole K, Khairy K, Friedrichs J, Franz C, Cisneros DA, Howard J, et al. Molecular-scale topographic cues induce the orientation and directional movement of fibroblasts on two-dimensional collagen surfaces. J Mol Biol 2005; 349:380 - 6; http://dx.doi.org/10.1016/j.jmb.2005.03.064; PMID: 15890202
- Franz CM, Muller DJ. Studying collagen self-assembly by time-lapse high-resolution atomic force microscopy. Methods Mol Biol 2011; 736:97 - 107; http://dx.doi.org/10.1007/978-1-61779-105-5_7; PMID: 21660723
- Friedl P, Maaser K, Klein CE, Niggemann B, Krohne G, Zänker KS. Migration of highly aggressive MV3 melanoma cells in 3-dimensional collagen lattices results in local matrix reorganization and shedding of alpha2 and beta1 integrins and CD44. Cancer Res 1997; 57:2061 - 70; PMID: 9158006
- Maaser K, Wolf K, Klein CE, Niggemann B, Zänker KS, Bröcker EB, et al. Functional hierarchy of simultaneously expressed adhesion receptors: integrin alpha2beta1 but not CD44 mediates MV3 melanoma cell migration and matrix reorganization within three-dimensional hyaluronan-containing collagen matrices. Mol Biol Cell 1999; 10:3067 - 79; PMID: 10512851
- Hofmann UB, Westphal JR, Waas ET, Zendman AJ, Cornelissen IM, Ruiter DJ, et al. Matrix metalloproteinases in human melanoma cell lines and xenografts: increased expression of activated matrix metalloproteinase-2 (MMP-2) correlates with melanoma progression. Br J Cancer 1999; 81:774 - 82; http://dx.doi.org/10.1038/sj.bjc.6690763; PMID: 10555745
- Ludwig T, Ossig R, Graessel S, Wilhelmi M, Oberleithner H, Schneider SW. The electrical resistance breakdown assay determines the role of proteinases in tumor cell invasion. Am J Physiol Renal Physiol 2002; 283:F319 - 27; PMID: 12110516
- Ludwig T, Püttmann S, Bertram H, Tatenhorst L, Paulus W, Oberleithner H, et al. Functional measurement of local proteolytic activity in living cells of invasive and non-invasive tumors. J Cell Physiol 2005; 202:690 - 7; http://dx.doi.org/10.1002/jcp.20168; PMID: 15389570
- Ludwig T, Theissen SM, Morton MJ, Caplan MJ. The cytoplasmic tail dileucine motif LL572 determines the glycosylation pattern of membrane-type 1 matrix metalloproteinase. J Biol Chem 2008; 283:35410 - 8; http://dx.doi.org/10.1074/jbc.M801816200; PMID: 18955496
- Rozanov DV, Deryugina EI, Monosov EZ, Marchenko ND, Strongin AY. Aberrant, persistent inclusion into lipid rafts limits the tumorigenic function of membrane type-1 matrix metalloproteinase in malignant cells. Exp Cell Res 2004; 293:81 - 95; http://dx.doi.org/10.1016/j.yexcr.2003.10.006; PMID: 14729059
- Bravo-Cordero JJ, Marrero-Diaz R, Megías D, Genís L, García-Grande A, García MA, et al. MT1-MMP proinvasive activity is regulated by a novel Rab8-dependent exocytic pathway. EMBO J 2007; 26:1499 - 510; http://dx.doi.org/10.1038/sj.emboj.7601606; PMID: 17332756
- Fouchard J, Mitrossilis D, Asnacios A. Acto-myosin based response to stiffness and rigidity sensing. Cell Adh Migr 2011; 5:16 - 9; http://dx.doi.org/10.4161/cam.5.1.13281; PMID: 20818154
- Schnaeker EM, Ossig R, Ludwig T, Dreier R, Oberleithner H, Wilhelmi M, et al. Microtubule-dependent matrix metalloproteinase-2/matrix metalloproteinase-9 exocytosis: prerequisite in human melanoma cell invasion. Cancer Res 2004; 64:8924 - 31; http://dx.doi.org/10.1158/0008-5472.CAN-04-0324; PMID: 15604254