Abstract
Natural Killer (NK) cells and Cytotoxic T lymphocytes (CTL) are critical for the immune response against virus infections or transformed cells. They kill target cells via polarized exocytosis of lytic proteins from secretory lysosomes (SL). Rab27a and munc13-4 interact directly and are required for target cell killing. How they cooperate in the intricate degranulation process is not known. We identified critical residues in munc13-4 for rab27 interaction and tested binding mutants in several complementation assays. In a rat mast cell line we replaced endogenous munc13-4 with ectopically expressed munc13-4 constructs. Unlike wild type munc13-4, binding mutants fail to rescue β-hexosaminidase secretion. In accord, expression of binding mutants in CTL of Familial Hemophagocytic Lymphohistiocytosis type 3 patients, does not rescue CD107 appearance on the plasma membrane. Total Internal Reflection Fluorescence (TIRF) imaging shows that munc13-4*rab27a restricts motility of SL in the subapical cytoplasm. We propose that rab27*munc13-4 tethers SL to the plasma membrane, a requirement for formation of a cognate SNARE complex for fusion.
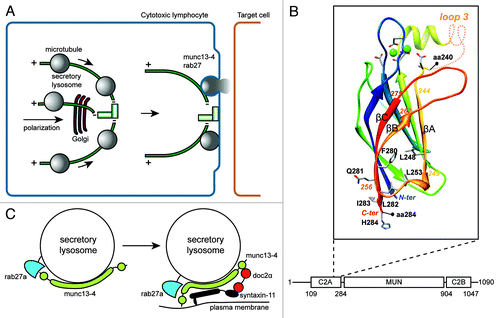
NK cells and CTL are crucial effectors of the innate and adaptive immune system, respectively. These cytotoxic lymphocytes destroy target cells through degranulation of lytic proteins from SL, a hybrid organelle with properties of lysosomes and secretory granules. Cytotoxic lymphocytes require external signals to release SL. Signaling in CTLs is initiated by the interaction of the T cell receptor with antigen loaded MHC class I protein on antigen presenting cells.Citation1 As a consequence of which the microtubules reorganize and the microtubule organizing center (MTOC) polarizes toward the immune synapse. 'Stronger' signals than needed for polarization, however are required to bring about the exocytic release process.Citation2 It is thought that the MTOC drags SL to the immune synapse during polarization where interactions of the MTOC with the plasma membrane set the stage for their fusion with and secretion () in the immune synapse.Citation5
Figure 1. Release mechanism of SL in CTL. (A) Antigen-dependent recognition of a target cell induces formation of a immune synapse between the two cell types. The CTL rapidly reorganizes its MTOC and the microtubule network toward this contact site. SL move in a minus-end direction along microtubules and cluster at the MTOC. Docking of the MTOC at the plasma membrane positions SL for fusion and lytic content release at the immune synapse. (B) Model of the 3D structure of the C2A domain of munc13–4 (aa 111–284). The model is based on the alignment of the C2A domain of human munc13–4 with the C2B domain of rat munc13–1, whose structure has been solved.Citation3 The limits of the 240–284 segment, including βA, βB, βC, are shown, as well as amino acids discussed in the text. Loops which were not modeled are represented by dashed lines. Amino acids of the Ca2+-binding site are shown, together with the bound Ca2+ ions (green spheres). (C) Hypothetical model for the function of munc13–4 rab27a complex. Immune signaling brings munc13–4 and rab27a in each other's vicinity. Colocalization facilitates the interaction between the two proteins and binding of rab27a could fold munc13–4 in a conformation that bridges SL membrane and plasma membrane.Citation4 The complex can also organize interactions with other proteins important for secretory lysosome release like syntaxin-11 and doc2α which then interacts with SNAP-23, establishing a regulatory circuit for controlling a cognate SNARE complex.
Familial Hemophagocytic Lymphohistiocytosis type 3 (FHL3) and Griscelli Syndrome 2 (GS2) patients are unable to clear viral infections because they lack functional munc13–4 and rab27a, respectively.Citation6 Ultrastructural analysis revealed that SL in FHL3 CTL do not fuse with the plasma membrane, suggesting that munc13–4 and rab27a are involved in a late stage of SL release.Citation7 The interaction between munc13–4 and rab27 and their localization on SL of rat basophil leukemia cells (RBL-2H3) is in agreement with such a role.Citation8 Several observations however do not seem to square with this scenario. First, munc13–4 contains autonomous membrane targeting information.Citation8 Second, rab27a and munc13–4 localize elsewhere than perforin and granzyme in resting cytotoxic lymphocytes.Citation9,Citation10 To understand the role of munc13–4 and rab27a complex, we developed precisely engineered munc13–4 point mutants that do not interact with rab27a and used these in functional complementation assays.Citation11
We show that munc13–4 contains a unique rab27 binding region that is distinct from the conserved rab27a binding sequence in other rab27 effectors. The rab27 interaction requires amino acid 240–290 which includes the last three β-strands strands (βA, βB and βC) of the C2A domain (aa 240–284) and a basic sequence (aa 285–290), linking the C2A domain to the following MUN domain (). Critical amino acids 280–285 are at the edge of C2A, where the βB strand may form a β-hairpin with βC-strand, which includes amino acids 280–285 (). The partners of the residues in βC which participate in the hydrophobic core of the C2A domain (F280, L282) are in strand βA (L248) and in the loop linking βA and βB (L253). A local “C2A-like” structure is therefore involved in binding of rab27a at the bottom of the C2A domain and of its C-terminal extension. This idea is supported by the fact that a similar region of the C2 domain is involved in munc13–1 C2A domain heterodimerization with the Zn-finger of RIM.Citation12
Munc13–4 point mutants that do not bind rab27a were introduced in CTLs from FHL3 patients that represent a human functional null allele. In contrast to wild type munc13–4, mutants were unable to restore fusion of SL with the plasma membrane, showing that the complex is essential in the pathway. Because SL are a hallmark of immune cells, we determined if other immune cells also require the munc13–4 rab27a complex in degranulation. We recapitulated the results obtained in the human FHL3 CTLs, using the RBL-2H3 mast cell line and complementation.Citation13 After knock down of endogenous munc13–4, immune receptor signaling failed to trigger degranulation as measured by release of content marker β-hexosaminidase. Expression of siRNA resistant wild type munc13–4 restores β-hexosaminidase exocytosis to wild type levels, but FHL3 mutants or rab27 binding mutants do not. Thus the munc13*4 rab27 complex also controls fusion of SL in immune cells that degranulate in a non-polarized manner. Munc13–4 regulates the merger of rab11 containing recycling endosomes and rab27a late endosomes.Citation9 This is a prerequisite for SL maturation in CTLs and as we show is a generic mechanism in immune cells. Importantly both in CTLs and in the mast cell line this function of munc13–4 does not involve rab27 because mutants deficient in rab27 binding behave identical as wild type munc13–4.Citation11
What could be the function of the munc13–4*rab27 complex? The abnormal distribution of SL at the immunological synapse is a hallmark of cells lacking functional rab27a or munc13–4, suggesting that the complex acts at a late stage of the release process.Citation7 Total Internal Fluorescence Reflection Fluorescence (TIRF) imaging of resting RBL-2H3 cells expressing cherry-rab27a and YFP-tagged munc13–4 constructs reveals motile SL in the plane of the plasma membrane and in and out of the TIRF zone, irrespective of the expressed munc13–4 rescue construct. After activation, SL became immobilized in the TIRF zone. The majority of SL (with markers cherrry-rab27a and YFP-tagged munc13–4 constructs) no longer shows long mobile tracks, which leads to a reduced caging area in activated cells. In contrast, munc13–4 mutants deficient in rab27 binding do not restrict mobility, showing that munc13–4 and rab27a act in the same pathway and that the interaction is necessary for tethering on the plasma membrane. Two other recent TIRF studies examined motility of SL immediately prior to fusion at the plasma membrane and arrived at similar conclusions. In neutrophils munc13–4 was found to limit mobilization of rab27a positive granules after lipopolysaccharide stimulation and to restrict them at the plasma membrane.Citation14 Complementing data in unstimulated NK cells show that rab27a provides motility for FasL containing SL at the plasma membrane.Citation15
How are the activities of munc13–4 and rab27a coordinated? We propose that binding of rab27a brings about a conformational change in munc13–4 that allows for tethering or priming of SL, two possibilities that are consistent with the available data. Immune signaling increases cytoplasmic Ca2+ which could induce phospholipid binding of the first C2 domain and its interaction with the nearby plasma membrane as suggested for neuronal munc13.Citation3 Subsequent interaction with rab27a releases the MUN domain from the SL membrane and extend it to the plasma membrane for association with doc2αCitation16 and SNAP23 (). Additional interactions with rab27 effector slp-2aCitation17,Citation18 cooperate to restrict the SL at the plasma membrane (not shown) and assist in SNARE complex formation. In accord with this idea are observations that the MUN domain can bind to syntaxinCitation19-Citation21 and contains homology with helical rod components of the CATCHR rab tethering complexes.Citation22,Citation23 In summary, SL release in immune cells appears to rely on similar molecules as synaptic vesicle exocytosis and granule exocytosis in endocrine cells. This suggests that a basic regulated secretory mechanism has evolved for which specificity and kinetic control is provided by cell type-specific molecules.
Acknowledgments
The study was supported by grants of the Dutch Cancer Society Koningin Wilhelmina Fonds (PvdS), the French National Institute for Health and Medical Research (INSERM), the French National Research Agency (ANR) and the Fondation pour la Recherche Médicale (FRM). NN is supported by a postdoctoral fellowship from l’Association de Recherche contre le cancer (ARC). We thank Rene Scriwanek for help with preparation of figures.
Conflict of interest disclosure
The authors declare no competing financial and scientific interests.
References
- Griffiths GM, Tsun A, Stinchcome JC. The immunological synapse: a focal point for endocytosis and exocytosis. J Cell Biol 2010; 189:399 - 406; http://dx.doi.org/10.1083/jcb.201002027; PMID: 20439993
- Jenkins MR, Tsun A, Stinchcombe JC, Griffiths GM. The strength of T cell receptor signal controls the polarization of cytotoxic machinery to the immunological synapse. Immunity 2009; 31:621 - 31; http://dx.doi.org/10.1016/j.immuni.2009.08.024; PMID: 19833087
- Higashio H, Nishimura N, Ishizaki H, Miyoshi J, Orita S, Sakane A, et al. Doc2α and munc13-4 regulate Ca2+ dependent secretory lysosome exocytosis in mast cells. J Immunol 2008; 180:4774 - 84; PMID: 18354201
- Araç D, Chen X, Khant HA, Ubach J, Ludtke SJ, Kikkawa M, et al. Close membrane-membrane proximity induced by Ca2+-dependent multivalent binding of synaptotagmin-1 to phospholipids. Nat Struct Mol Biol 2006; 13:209 - 17; http://dx.doi.org/10.1038/nsmb1056; PMID: 16491093
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 2006; 443:462 - 5; http://dx.doi.org/10.1038/nature05071; PMID: 17006514
- Pachlopnik Schmid J, Cote M, Menager MM, Burgess A, Nehme N, Menasche G, et al. Inherited defects in lymphocyte cytotoxic activity. Immunol Rev 2010; 235:10 - 23; PMID: 20536552
- Feldmann J, Callebaut I, Raposo G, Certain S, Bacq D, Dumont C, et al. Munc13-4 is essential for cytolytic granules fusion and is mutated in a form of Familial Hemophagocytic Lymphohistiocytosis (FHL3). Cell 2003; 115:461 - 73; http://dx.doi.org/10.1016/S0092-8674(03)00855-9; PMID: 14622600
- Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CH, van Loon A, et al. Munc13-4 is and effector of rab27a and controls secretion of lysosomes in haematopoietic cells. Mol Biol Cell 2005; 16:731 - 41; http://dx.doi.org/10.1091/mbc.E04-10-0923; PMID: 15548590
- Ménager MM, Menasche M, Ramoa M, Knapnougel P, Ho CH, Garfa M, et al. Secretory cytotoxic granule maturation and exocytosis require the effector protein hMunc13-4. Nat Immunol 2007; 8:257 - 67; http://dx.doi.org/10.1038/ni1431; PMID: 17237785
- Wood SM, Meeths M, Chiang SCC, Bechensteen AG, Boelens JJ, Heilmann C, et al. Different NK cell activating receptors preferentially recruit rab27a or munc13-4 to perforin containing granules for cytotoxicity. Blood 2009; 114:4117 - 27; http://dx.doi.org/10.1182/blood-2009-06-225359; PMID: 19704116
- Elstak ED, Neeft M, Nehme NT, Voortman J, Cheung M, Goodarzifard M, et al. Munc13-4 rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood 2011; 118:1570 - 8; http://dx.doi.org/10.1182/blood-2011-02-339523; PMID: 21693760
- Lu J, Machius M, Dulubova I, Dai H, Südhof TC, Tomchick DR, et al. Structural basis for a munc13-1 homodimer to munc13-1/RIM heterodimer switch. PLoS Biol 2006; 4:e192; http://dx.doi.org/10.1371/journal.pbio.0040192; PMID: 16732694
- Elstak E, de Jong A, van der Sluijs P. A platform for complementation and characterization of familial haemophagocytic lymphohistiocytosis 3 mutations. J Immunol Methods 2011; 365:58 - 66; http://dx.doi.org/10.1016/j.jim.2010.12.009; PMID: 21182842
- Johnson JL, Hong H, Monfregola J, Kiosses WB, Catz SD. Munc13-4 restricts motility of rab27a expressing vesicles to facilitate lipopolysaccharide-induced priming of exocytosis in neutrophils. J Biol Chem 2011; 286:5647 - 56; http://dx.doi.org/10.1074/jbc.M110.184762; PMID: 21148308
- Liu D, Meckel T, Long EO. Distinct role of rab27a in granule movement at the plasma membrane and in the cytosol of NK cells. PLoS ONE 2010; 5:e12870; http://dx.doi.org/10.1371/journal.pone.0012870; PMID: 20877725
- Ménasché G, Menager MM, Lefebre JM, Deutsch E, Althman R, Klambert N, et al. A newly identified isoform of slp2-a associates with rab27a in cytotoxic T cells and participates to cytotoxic T cell degranulation. Blood 2008; 112:5052 - 62; http://dx.doi.org/10.1182/blood-2008-02-141069; PMID: 18812475
- Holt O, Kanno E, Bossi G, Booth S, Daniele T, Santoro A, et al. Slp1 and slp2-a localize to the plasma membrane of CTL and contribute to secretion at the immunological synapse. Traffic 2008; 9:446 - 57; http://dx.doi.org/10.1111/j.1600-0854.2008.00714.x; PMID: 18266782
- Betz A, Okamoto M, Benseler F, Brose N. Direct interaction of the rat unc-13 homologue munc13-1 with the N terminus of syntaxin. J Biol Chem 1997; 272:2520 - 6; http://dx.doi.org/10.1074/jbc.272.4.2520; PMID: 8999968
- Khodthong C, Kabachinski G, James DJ, Martin TFJ. Munc13 homology domain-1 caps/unc31 mediates SNARE binding required for priming vesicle exocytosis. Cell Metab 2011; 14:254 - 63; http://dx.doi.org/10.1016/j.cmet.2011.07.002; PMID: 21803295
- Ma C, Li W, Xu Y, Rizo J. Munc13 mediates the transition from the closed syntaxin-munc18 complex to the SNARE complex. Nat Struct Mol Biol 2011; 18:542 - 9; http://dx.doi.org/10.1038/nsmb.2047; PMID: 21499244
- Pei J, Ma C, Rizo J, Grishin NV. Remote homology between munc13 MUN domain and vesicle tethering complexes. J Mol Biol 2009; 391:509 - 17; http://dx.doi.org/10.1016/j.jmb.2009.06.054; PMID: 19563813
- Hughson FM, Reinisch K. Structure and mechanism in membrane trafficking. Curr Opin Cell Biol 2010; 22:454 - 60; http://dx.doi.org/10.1016/j.ceb.2010.03.011; PMID: 20418086
- Shin OH, Lu J, Rhee JS, Tomchick DR, Pang ZP, Wojcik SM, et al. Munc13 C2B domain is an activity-dependent Ca2+ regulator of synaptic exocytosis. Nat Struct Mol Biol 2010; 17:280 - 8; http://dx.doi.org/10.1038/nsmb.1758; PMID: 20154707