Abstract
The release of hormones and neurotransmitters from vesicles can be modified by the regulation of the fusion pore, an aqueous channel that forms upon the fusion of the vesicle membrane with the plasma membrane. However, the mechanisms are unclear. Munc18–1 protein interacts with Syntaxin1 (Synt 1), a member of the SNARE proteins, which plays an important role in exocytosis. It has been shown that Munc18–1 has multiple roles, both in pre- and post-fusion stages of exocytosis. It regulates the traffic of Synt1 to the plasma membrane. By inhibiting the tethering of the vesicle SNARE protein Synaptobrevin 2 (Syb2) solely to Synt1 at the plasma membrane, but favoring the vesicular tethering to the preformed binary cis SNARE complex of Synt1A-SNAP25B, Munc18–1 is tuning vesicle docking and the membrane merger process. Additionally, Munc18–1 affects exocytosis at the post-fusion stage by regulating the fusion pore properties (i.e., dwell-time and fusion pore diameter). Among many possible mechanisms that may regulate the fusion pore, but have never been considered previously, is the influence of Munc18–1 on the membrane anisotropy, which determines the local spontaneous membrane curvature and the architecture of the fusion pore. We here propose that Munc18–1 affects the fusion pore by modulating the dynamic local (re)arrangement of anisotropic membrane components within the highly curved fusion pore nanostructure, to which proteins, lipids or their complexes can participate.
Regulated exocytosis consists of sequential steps, such as secretory vesicle delivery and docking to the plasma membrane, leading to the fusion of the vesicle and the plasma membranes. The proteins which play an important role in the tethering/docking and fusion are members of the SNARE proteins (Soluble NSF Attachment Protein Receptor proteins), which form a complex; Synaptobrevin2 (Syb2) on vesicles and Syntaxin1 (Synt1) and Synaptosome-Associated Protein (SNAP25) on the plasma membrane.Citation1 It has been proposed, that a small number of SNARE complexes is sufficient for membrane fusion.Citation2,Citation3 However, additional proteins are required to fine-tune and increase the speed of this process. Such proteins are Sec/Munc18 (SM) proteins, which are, apart from SNAREs, the only indispensable proteins for exocytosis in all species, from yeast to mammals.Citation4
The role of Munc18–1 in pre-fusion steps of exocytosis
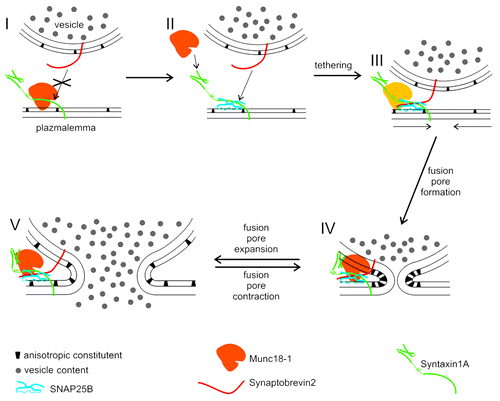
Munc18–1, which is the neuronal isoform of SM proteins, has multiple roles already in pre-fusion steps. First, Munc18–1 regulates the traffic of vesicles since in Munc18–1 deficient cells fewer vesicles approach the target membrane.Citation5 Second, it was proposed that Munc18–1, bound to Synt1A, interacts with SNAP25B and Syb2 to initiate the formation of the ternary SNARE complex, which ensures tethering/docking of vesicles.Citation6 The step after the formation of the ternary SNARE complex is more enigmatic; Munc18–1 is thought to either detach from the SNARE complex,Citation6,Citation7 or remain present to form a hypercomplex.Citation4,Citation7,Citation8 However, recent combined experiments where the nano-mechanics of protein-protein interactions by atomic force microscopyCitation9 and the function of a single fusion pore was studied by the high-resolution membrane capacitance measurements,Citation10 have clearly shown that Munc18–1 affects the sequence of steps leading to the formation of the ternary SNARE complex and that this has pre- and post-fusion effects on exocytosis.Citation11 Apparently, there are two modes of vesicle-plasma membrane interactions depending on the presence of cytosolic Munc18–1 within the cell. The presence of Munc18–1 would favor the formation of Synt1A-SNAP25B cis complex at the plasma membrane with the vesicular Syb2 (). In the absence of Munc18–1, a higher proportion of vesicle-plasma membrane interaction would base on Synt1A-Syb2 interactions. However, the interaction of Syb2 with the Synt1A+SNAP25B cis complex in the presence/absence of Munc18–1 (Fig. 1II, III) would lead to a more stable tethering compared with the tethering based on Synt1A-Syb2 interaction alone.Citation9
Figure 1. A model describing the role of Munc18–1, SNARE proteins and anisotropic constituents in vesicle fusion. Munc18–1 binds Syntaxin1A in a closed conformation, which prevents the formation of Synaptobrevin2–Syntaxin1A trans interaction (I). Synaptobrevin2, however, can interact with cis complex of Syntaxin1A and SNAP25 to form the ternary SNARE complex (II, III). The ternary SNARE complex enables stable tethering of vesicles to the plasma membrane, either in presence or absence (thus lighter appearance) of Munc18–1 (III). The fusion of vesicle and plasma membranes is facilitated by the effect of Synaptotagmin1 (not shown in the Fig.) on the rearrangement of anisotopic constituents (III, arrows) (Lai et al., 2011). Initially, the fusion pore is narrow and stable (IV), due to the locally high concentration of anisotropic constituents (Jorgačevski et al., 2010). Fusion pore expansion and contraction (IV, V) are affected by the presence of Munc18–1 (Jorgačevski et al., 2011), possibly also by modulating the dissipation of anisotropic constituents (V).
The role of Munc18–1 in vesicle fusion and post-fusion steps of exocytosis
Tethering/docking of vesicles is followed by the fusion of the vesicle membrane with the plasma membrane. This process results in the formation of the fusion pore, an aqueous channel, through which the vesicle content is released.Citation12 The fusion pore was considered to be a short-lived meta-stable state, which is quickly subject to widening and eventually leading to the complete merger of vesicle and plasma membranes.Citation13 Recent studies, however, have expanded this model. It is now clear, that in the majority of cell types there is an additional mode of vesicle content discharge where the diameter of the fusion pores fluctuates between an open and a virtually closed state.Citation11,Citation14-Citation19 Moreover, the diameter and the duration of the open fusion pore state depend on stimulation.Citation16,Citation20 Additionally, the stable fusion pore diameter depends also on the vesicle diameter.Citation17 However, the molecular events mediating the transitions between discrete open states of a fusion pore are unclear.
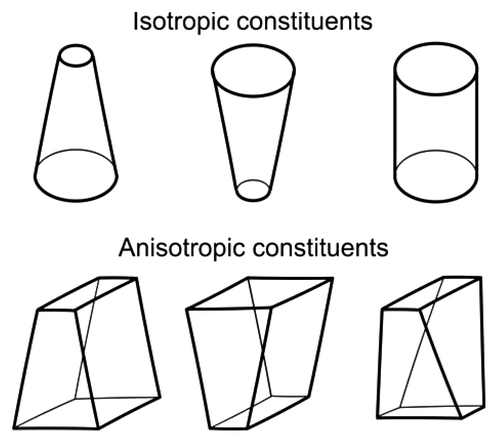
A growing body of evidence now points to the Munc18–1 as an important regulator of the fusion pore state transitions.Citation11,Citation21-Citation23 It is reasonable to assume that this regulation is mediated through Synt1, the binding partner of Munc18–1 which has the most widely recognized role in exocytosis.Citation6 However, other possibilities cannot be excluded. For instance, it was recently shown, that synaptotagmin1 affects the stability of lipid bilayer by phase separating the phosphatidylserine which induces a positive curvature of lipid bilayers.Citation24 The region of the fusion pore, on the other hand, is stabilized by anisotropic constituents (lipids, proteins or their complexes), which confer a negative curvature (Fig. 1IV andCitation17). Anisotropic membrane components are, unlike isotropic constituents, characterized by non-axisymetric structure (see for comparison of anisotropic and isotropic constituents) which is essential for forming subnanometer diameter fusion pores.Citation17 The presence of Munc18–1 may directly or indirectly affect the local membrane anisotropy in the neck of the fusion pore, by modulating the accumulation of anisotropic constituents after the fusion pore is formed (Fig. 1IV). If Munc18–1 would dissociate from the SNARE complex, when this is disassembled following its formation,Citation25 this may lead to the rearrangement of anisotropic constituents in the neck of the fusion pore, hence reducing the spontaneous curvature of the fusion pore. Reduced spontaneous curvature of the fusion pore would decrease its stability, and thus promote the widening of the fusion pore diameter ().
To conclude, Munc18–1 is a protein ubiquitously participating in practically all exocytotic steps. The role of Munc18–1 has been traditionally evaluated through the prism of its interactions with other proteins, while the interactions of Munc18–1 with lipids remain unexplored. Therefore one of the future goals should be assessing these interactions, which can prove critically important for the overall understanding of Munc18–1 functions in exocytosis.
References
- Jahn R, Lang T, Südhof T. Membrane fusion. Cell 2003; 112:519 - 33; http://dx.doi.org/10.1016/S0092-8674(03)00112-0; PMID: 12600315
- van den Bogaart G, Holt MG, Bunt G, Riedel D, Wouters FS, Jahn R. One SNARE complex is sufficient for membrane fusion. Nat Struct Mol Biol 2010; 17:358 - 64; http://dx.doi.org/10.1038/nsmb.1748; PMID: 20139985
- Mohrmann R, de Wit H, Verhage M, Neher E, Sørensen JB. Fast vesicle fusion in living cells requires at least three SNARE complexes. Science 2010; 330:502 - 5; http://dx.doi.org/10.1126/science.1193134; PMID: 20847232
- Toonen RF, Verhage M. Munc18-1 in secretion: lonely Munc joins SNARE team and takes control. Trends Neurosci 2007; 30:564 - 72; http://dx.doi.org/10.1016/j.tins.2007.08.008; PMID: 17956762
- Toonen RF, Kochubey O, de Wit H, Gulyas-Kovacs A, Konijnenburg B, Sørensen JB, et al. Dissecting docking and tethering of secretory vesicles at the target membrane. EMBO J 2006; 25:3725 - 37; http://dx.doi.org/10.1038/sj.emboj.7601256; PMID: 16902411
- Misura KM, Scheller RH, Weis WI. Three-dimensional structure of the neuronal-Sec1-syntaxin 1a complex. Nature 2000; 404:355 - 62; http://dx.doi.org/10.1038/35006120; PMID: 10746715
- Zilly FE, Sorensen JB, Jahn R, Lang T. Munc18-bound syntaxin readily forms SNARE complexes with synaptobrevin in native plasma membranes. PLoS Biol 2006; 4:e330; http://dx.doi.org/10.1371/journal.pbio.0040330; PMID: 17002520
- Südhof TC, Rothman JE. Membrane fusion: grappling with SNARE and SM proteins. Science 2009; 323:474 - 7; http://dx.doi.org/10.1126/science.1161748; PMID: 19164740
- Liu W, Montana V, Bai J, Chapman E, Mohideen U, Parpura V. Single molecule mechanical probing of the SNARE protein interactions. Biophys J 2006; 91:744 - 58; http://dx.doi.org/10.1529/biophysj.105.073312; PMID: 16648158
- Kreft M, Zorec R. Cell-attached measurements of attofarad capacitance steps in rat melanotrophs. Pflugers Arch 1997; 434:212 - 4; http://dx.doi.org/10.1007/s004240050387; PMID: 9136678
- Jorgačevski J, Potokar M, Grilc S, Kreft M, Liu W, Barclay JW, et al. Munc18-1 tuning of vesicle merger and fusion pore properties. J Neurosci 2011; 31:9055 - 66; http://dx.doi.org/10.1523/JNEUROSCI.0185-11.2011; PMID: 21677188
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev 2003; 83:581 - 632; PMID: 12663867
- Spruce AE, Breckenridge L, Lee A, Almers W. Properties of the fusion pore that forms during exocytosis of a mast cell secretory vesicle. Neuron 1990; 4:643 - 54; http://dx.doi.org/10.1016/0896-6273(90)90192-I; PMID: 2344404
- Stenovec M, Kreft M, Poberaj I, Betz W, Zorec R. Slow spontaneous secretion from single large dense-core vesicles monitored in neuroendocrine cells. FASEB J 2004; 18:1270 - 2; PMID: 15180959
- Elhamdani A, Azizi F, Artalejo CR. Double patch clamp reveals that transient fusion (kiss-and-run) is a major mechanism of secretion in calf adrenal chromaffin cells: high calcium shifts the mechanism from kiss-and-run to complete fusion. J Neurosci 2006; 26:3030 - 6; http://dx.doi.org/10.1523/JNEUROSCI.5275-05.2006; PMID: 16540581
- Vardjan N, Stenovec M, Jorgacevski J, Kreft M, Zorec R. Subnanometer fusion pores in spontaneous exocytosis of peptidergic vesicles. J Neurosci 2007; 27:4737 - 46; http://dx.doi.org/10.1523/JNEUROSCI.0351-07.2007; PMID: 17460086
- Jorgačevski J, Fosnaric M, Vardjan N, Stenovec M, Potokar M, Kreft M, et al. Fusion pore stability of peptidergic vesicles. Mol Membr Biol 2010; 27:65 - 80; http://dx.doi.org/10.3109/09687681003597104; PMID: 20334578
- Bandmann V, Kreft M, Homann U. Modes of exocytotic and endocytotic events in tobacco BY-2 protoplasts. Mol Plant 2011; 4:241 - 51; http://dx.doi.org/10.1093/mp/ssq072; PMID: 21135068
- Malarkey EB, Parpura V. Temporal characteristics of vesicular fusion in astrocytes: examination of synaptobrevin 2-laden vesicles at single vesicle resolution. J Physiol 2011; 589:4271 - 300; PMID: 21746780
- Jorgačevski J, Stenovec M, Kreft M, Bajić A, Rituper B, Vardjan N, et al. Hypotonicity and peptide discharge from a single vesicle. Am J Physiol Cell Physiol 2008; 295:C624 - 31; http://dx.doi.org/10.1152/ajpcell.00303.2008; PMID: 18632733
- Fisher RJ, Pevsner J, Burgoyne R. Control of fusion pore dynamics during exocytosis by Munc18. Science 2001; 291:875 - 8; http://dx.doi.org/10.1126/science.291.5505.875; PMID: 11157167
- Ciufo LF, Barclay J, Burgoyne R, Morgan A. Munc18-1 regulates early and late stages of exocytosis via syntaxin-independent protein interactions. Mol Biol Cell 2005; 16:470 - 82; http://dx.doi.org/10.1091/mbc.E04-08-0685; PMID: 15563604
- Graham ME, Handley M, Barclay J, Ciufo L, Barrow S, Morgan A, et al. A gain-of-function mutant of Munc18-1 stimulates secretory granule recruitment and exocytosis and reveals a direct interaction of Munc18-1 with Rab3. Biochem J 2008; 409:407 - 16; http://dx.doi.org/10.1042/BJ20071094; PMID: 17919117
- Lai AL, Tamm LK, Ellena JF, Cafiso DS. Synaptotagmin 1 modulates lipid acyl chain order in lipid bilayers by demixing phosphatidylserine. J Biol Chem 2011; 286:25291 - 300; http://dx.doi.org/10.1074/jbc.M111.258848; PMID: 21610074
- An SJ, Grabner C, Zenisek D. Real-time visualization of complexin during single exocytic events. Nat Neurosci 2010; 13:577 - 83; http://dx.doi.org/10.1038/nn.2532; PMID: 20383135