Abstract
We previously reported that mitotic endoplasmic reticulum (ER) membrane cisternae or sheets directly assemble mammalian nuclear envelope (NE) at the end of mitosis. In this study, we investigated the dynamics of the high curvature regions of partially assembled nuclear envelope membrane using reticulon4a as a probe. We found that, after sorting out reticulon4a from the nascent NE membrane sheets, reticulon4a is specifically localized to the leading edges. Our 3D time lapse images suggested that ER tubules could be incompetent in assembling the NE membrane. Our findings suggest a possible role of reticulons at the leading edges during the NE re-assembly and provide further evidences that the mitotic assembly of NE is by ER cisternae rather than tubules.
Keywords: :
During mitosis, the NE collapses and merges with the mitotic ER. At the end of mitosis, NE re-assembles around the chromosome mass from the mitotic ER.Citation1 From both live cell imaging and electron tomography, we recently provided extensive evidences to show that the mitotic ER is predominantly cisternal or sheet during mitosisCitation2 and the formation of NE is by the direct wrapping and migration of the ER cisternae around the chromosome mass.Citation3 It is known that the affinity between NE membrane protein and chromatin DNA is presumably the major driving force for the adherence and migration of ER cisternae on the chromosome mass.Citation4,Citation5 However, we are still lacking the detailed molecular picture of the NE assembly.
Reticulon4a (Rtn4) belongs to the reticulon/DP1 family ER membrane proteins which are distributed preferentially to highly curved ER membrane regions, such as tubules and rims of sheets.Citation6 To further explore the dynamic process of the NE assembly, we used GFP-Rtn4 to probe the high curvature regions of the newly assembled NE. Live HeLa cells expressing GFP-Rtn4 and mCherry-Sec61β were subjected to fast dual-color 3D imaging using spinning disk confocal as described previously.Citation3 In , panel A shows an early telophase cell with partially assembled NE. At periphery, Sec61β and Rtn4 co-localize at the curvy-linear membrane profiles, which correspond to the mitotic ER cisternae (, filled arrowheads).Citation2 Around the chromosome masses, Rtn4 is depleted from the nascent NE membrane while Sec61β remains the same intensity as the mitotic ER (, open arrowheads). A close examination of image stacks revealed that Rtn4 appears as spots joining the ends of the curvy-linear Sec61β profiles on continuous optical sections (, filled arrowheads).These spots should correspond to the orthogonal optical sections of leading edges of the nascent NE membrane sheets in 3D. There are also some Rtn4 positive puncta and lines co-localizing with the nascent NE membrane profiles (, filled arrowheads). Serial sections reveal that these profiles should be the tangential optical sections of the leading edges. In dual-color 2D time lapse imaging, we observed that the leading edge localization of Rtn4 is maintained during the dynamic migration of the nascent NE until the completion of the NE ().
Figure 1. The distribution of Rtn4 in the high curvature regions of nascent NE. (A) A 3D image stack was acquired from a HeLa cell expressing GFP-Rtn4 and mCherry-Sec61β during the mitotic NE assembly and subsequently deconvolved.Citation3 The middle section of the cell is shown. Filled arrowheads, peripheral ER cisternae; open arrowheads, nascent NE. Bar, 10 µm. In “merge,” the boxed regions are selected and series of image sections above or below are shown. (B) Rtn4 positive spots (filled arrowheads) localized at the ends of curvy-linear ER membrane profiles. Bar, 5 µm. (C) Rtn4 positive line profiles co-localized with the curvy-linear ER membrane profiles. Bar, 5 µm. (D) The edge localization of Rtn4a during the dynamic assembly of the NE. A 2D time-lapse was acquired from a HeLa cell expressing GFP-Rtn4 and mCherry-Sec61β during the mitotic NE assembly. Selected frames are displayed. Bar, 5 µm. (E) A Rtn4 positive tubule (filled arrowheads) that is originating from the nascent NE and showing no apparent connection with ER cisternae. Bar, 2 µm. In (B, C and E), sections corresponding to (A) is 0.00 µm. Note that in (C), the series of sections are below the boxed regions of (A).
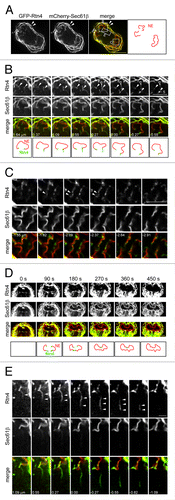
These observations correspond well to the property of Rtn4 as a high curvature sensor and verify the usage of Rtn4 as a marker to probe high curvature regions of the nascent NE membrane. Occasionally, Rtn4 could be found deep inside the nascent NE membrane, which does not apparently correspond to either leading migrating edge or tubules (, open arrowheads). We think they are probably the edges of the closely opposed NE membrane sheets that are not yet fused. A supporting example is illustrated in the images from 180 to 360 sec in . When the migrating leading edges meet and are in the process of fusion, Rtn4 is observed to remain as a dot (). That very few such sites of Rtn4 could be observed suggests that the nascent NE could be an intact membrane sheet with very few gaps. Our observations therefore demonstrated that, during the assembly of NE, reticulons re-distribute from nascent NE membrane sheets to the leading edges, suggesting that reticulons could be essential for stabilizing the edges of the nascent NE membrane sheets.
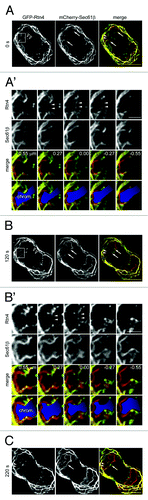
In addition to edge localization, Rtn4 were also found on the membrane tubules around the chromosome mass as shown in . These tubules are very weakly labeled by Sec61β, stressing the importance of using Rtn4 to unambiguously track tubules. Examination of image stacks revealed that they originate from the nascent NE membrane and do not appear to connect to peripheral ER cisternae (, filled arrowheads). Previously, the finding of ER tubules around chromosome mass was used as in vivo evidence to support a model in which ER tubules assemble NE.Citation7 However, it was unclear whether these chromosome proximal ER tubules are capable of forming NE. We decided to monitor the ER tubules around the chromosome mass to see if they contribute to the NE assembly. In , a single HeLa cell expressing GFP-Rtn4 and mCherry-Sec61β was imaged at three time points—0 (), 120 () and 220 sec (). Most ER tubules are pointing away from the chromosome mass and dynamically appear and disappear ( and B, open arrowheads). We focused on an ER tubule that seems to penetrate across the chromosome mass and make connections between two sheets of the NE membrane (’, filled arrowheads). After 120 sec, the tubule remains at the same location and does not assemble NE membrane sheets as shown by serial sections (’, filled arrowheads). In contrast, during the same period, the Sec61β labeled nascent NE membrane sheets migrate further toward the center (, arrows), indicating that the NE assembly is permissive during this period. It is possible that the overexpression of GFP-Rtn4 could result in the incapability of tubules in assembling NE. However, we could argue that ER tubules are highly enriched in reticulons even without the expression of exogenous reticulons.Citation6 Therefore, our observation suggested that ER tubules could be incapable of assembling NE membrane sheets in vivo. Collectively, our imaging data provide further evidences that the assembly of NE membrane is directly from the wrapping and migration of mitotic ER cisternae rather than the attachment and lateral expansion of mitotic ER tubules.
Figure 2. Evidence showing that tubules around the chromosome mass do not assemble the NE. Dual-color 3D images were acquired from a single HeLa cell expressing GFP-Rtn4 and mCherry-Sec61β during the mitotic NE assembly at 0 (A), 120 (B) and 220 sec (C). The boxed regions in (A and B) are selected and the image series above and below are shown in (A’ and B’), respectively. Sections corresponding to (A and B) are 0.00 µm. In lower rows of A’ and B’, the chromosome masses (chrom.) are manually traced and shaded as blue according to the contours of the nascent NE membrane. The same tubule (marked by filled arrowheads) is continuously present within the chromosome mass for at least 220 sec without being converted to the NE. Open arrowheads, dynamic tubules; arrows, the migrating fronts of the nascent NE. Bars, 10 µm (A, B and C) and 5 µm (A’ and B’). Note that panels from and are from the same set of data.
| Abbreviations: | ||
| ER | = | Endoplasmic reticulum |
| NE | = | nuclear envelope |
| Rtn | = | reticulon |
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Tom Rapoport for providing GFP-Reticulon4a. This work was supported by NIH grants GM-075252 (to T.K.), U54 AI057159 (New England Regional Center of Excellence in Biodefense and Emerging Infectious Disease, Core Imaging Facility) (to T.K.) and SBS SUG M58080013 (to L.L.).
References
- Hetzer MW, Wente SR. Border control at the nucleus: biogenesis and organization of the nuclear membrane and pore complexes. Dev Cell 2009; 17:606 - 16; http://dx.doi.org/10.1016/j.devcel.2009.10.007; PMID: 19922866
- Lu L, Ladinsky MS, Kirchhausen T. Cisternal organization of the endoplasmic reticulum during mitosis. Mol Biol Cell 2009; 20:3471 - 80; http://dx.doi.org/10.1091/mbc.E09-04-0327; PMID: 19494040
- Lu L, Ladinsky MS, Kirchhausen T. Formation of the postmitotic nuclear envelope from extended ER cisternae precedes nuclear pore assembly. J Cell Biol 2011; 194:425 - 40; http://dx.doi.org/10.1083/jcb.201012063; PMID: 21825076
- Ulbert S, Platani M, Boue S, Mattaj IW. Direct membrane protein-DNA interactions required early in nuclear envelope assembly. J Cell Biol 2006; 173:469 - 76; http://dx.doi.org/10.1083/jcb.200512078; PMID: 16717124
- Anderson DJ, Vargas JD, Hsiao JP, Hetzer MW. Recruitment of functionally distinct membrane proteins to chromatin mediates nuclear envelope formation in vivo. J Cell Biol 2009; 186:183 - 91; http://dx.doi.org/10.1083/jcb.200901106; PMID: 19620630
- Voeltz GK, Prinz WA, Shibata Y, Rist JM, Rapoport TA. A class of membrane proteins shaping the tubular endoplasmic reticulum. Cell 2006; 124:573 - 86; http://dx.doi.org/10.1016/j.cell.2005.11.047; PMID: 16469703
- Anderson DJ, Hetzer MW. Reshaping of the endoplasmic reticulum limits the rate for nuclear envelope formation. J Cell Biol 2008; 182:911 - 24; http://dx.doi.org/10.1083/jcb.200805140; PMID: 18779370