Abstract
Gas vesicles are gas-filled microcompartments produced by many cyanobacteria and haloarchaea to regulate buoyancy and control positioning in the water column. Recently we identified the first case of gas vesicle production by a member of the Enterobacteriaceae, Serratia sp ATCC39006. Gas vesicle production enabled colonisation of the air-liquid interface and was positively regulated in low-oxygen conditions, suggesting development of these intracellular organelles is an adpative mechanism facilitating migration to the water surface. Vesicle production was also regulated by the intercellular communication molecule N‑butanoyl-L‑homoserine lactone (BHL) showing that gas vesicle production is controlled at the population level, through quorum sensing, with BHL acting as a morphogen. Gas vesicle production was also reciprocally regulated with flagella-driven swarming motility by the global regulatory protein RsmA, suggesting a fork in the regulatory pathway that controls induction of these distinct modes of mobility. Here we discuss these findings in the context of the interesting physiology of Serratia 39006 and highlight future prospects for gas vesicle research in this highly tractable strain.
While many bacteria use energy-powered flagella to propel themselves into favorable niches in response to environmental cues, others have evolved a more passive approach to movement. Several aquatic species, particularly cyanobacteria and haloarchaea, instead regulate their buoyancy, allowing them to float upwards or stabilize their position in the water column.Citation1 Positive buoyancy in these species is achieved through the production of gas-filled organelles called gas vesicles (GV) (or, as conglomerates, gas vacuoles). This GV‑driven buoyancy is responsible for the flotation of many photosynthetic cyanobacteria commonly observed as “toxic blooms” in stagnant waters.Citation2
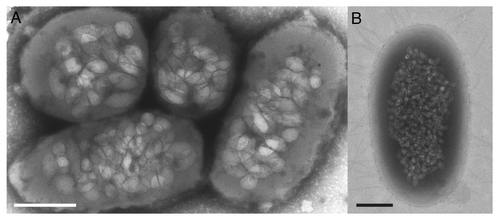
We recently reported the first discovery of natural GV production by a member of the Enterobacteriaceae, Serratia sp ATCC 39006 (Serratia 39006).Citation3 Serratia 39006 was originally isolated from a New Jersey salt marsh and initially studied for its ability to produce a broad spectrum β‑lactam antibiotic of the carbapenem class.Citation4 It also produces the red-pigmented linear tripyrolle, prodigiosin, currently studied for its immunosuppressive properties and cytotoxic activity against cancer cells.Citation5 This species also performs distinct modes of flagella-driven movement, including swimming and swarming motility.Citation6 Our discoveries revealed that GV production by this strain enabled colonisation of the air-liquid interface, suggesting that this bacterium utilizes these vesicles to facilitate access to the oxygen-rich water surface. Consistent with this hypothesis, Serratia 39006 gas vesicle morphogenesis was activated in cultures with reduced aeration.
Gas vesicles are gas-filled intracellular protein cylinders with conical ends, mostly comprised of a single structural subunit, GvpA, a protein common to all gas vesicle-producing species discovered, thus far.Citation1 Different species produce different variants of GvpA (and the strengthening protein GvpC), generating GVs with varied widths, volumes and critical collapse pressures. Evidence indicates these parameters are a constant target of natural selection, as fatter vesicles are less costly to make (relative to the amount of gas occupancy), while narrower vesicles are stronger and more resistant to pressure-induced collapse.Citation7-Citation9 Serratia 39006 encodes a ~17‑kb region of DNA necessary and sufficient for GV morphogenesis, confirmed both through mutagenesis and the genetic engineering of buoyant, gas vesicle-expressing Escherichia coli. Serratia 39006 encodes three distinct homologs of GvpA and was able to produce vesicles of different widths, suggesting that it may be able to regulate vesicular dimensions, perhaps as a physiological and developmental response to environmental conditions.
Bacteria commonly use diffusible molecule-based cell-cell signaling mechanisms to coordinate behaviors at the population level. This type of communication is often termed “quorum sensing,” because the concentration of the relevant signaling molecule(s) is often a reflection of bacterial cell density. This apparently social behavior can be advantageous to a bacterium, especially if the phenotypes controlled by the intercellular signaling molecule(s) are most effective when expressed simultaneously.Citation10 In Serratia 39006, several phenotypes are controlled by quorum sensing, including swarming motility and the production of prodigiosin and a carbapenem.Citation6,Citation11 We found that a mutant unable to produce the quorum sensing signaling molecule, N‑butanoyl-L‑homoserine lactone (BHL) was also unable to form GVs or float. Supplementation with exogenous BHL restored these phenotypes, indicating gas vesicle synthesis was dependent on, and positively regulated by, quorum sensing. Thus, in this bacterial strain, BHL acts as a morphogen, stimulating gas vesicle biogenesis throughout the cell population.
What advantages might be accrued by linking quorum sensing with the developmental assembly of GVs? One rationale for this cell density-dependent flotation phenomenon, is that Serratia 39006 may be able to increase its floating velocity, through cell-cell association at high cell density. The floating velocity of an object in solution is a function of both relative density and the radius of the suspended object squared (Stoke’s law), so GV development may only be really effective as a means of migrating to the surface, if cells float together. For example, it has been shown that large colonies (~3 mm diameter) of gas vacuolated Microcystis can float with velocities of over 1 mm.s−1,Citation12 while single cyanobacterial cells float at only 2 µm.s−1.Citation1 Interestingly, to put these speeds in context, most flagella driven motion only propels in the order of 30 µm.s−1.Citation13 Therefore, while gas vesicle driven movement may be limited to upward motion, it has some clear advantages, both for economy and speed.Citation1
We also found that a mutation inactivating the small-RNA binding regulator, RsmA, resulted in inactivation of GV production. RsmA and its E. coli ortholog CsrA, control the expression of genes involved in secondary metabolism and particularly carbohydrate storage; in E. coli mutants defective in csrA overproduce glycogen to the point of lethality.Citation14 Interestingly, in our experiments the rsmA mutant strain appeared to sink even more quickly than a strain lacking the GV genes, suggesting RsmA may control additional buoyancy factors. It is possible that, in the rsmA mutant, carbon stores act as ballast, as in the photosynthetic cyanobacteria, where buoyant, highly irradiated cells accumulate carbohydrate stores and sink, resulting in a diel pattern of buoyancy change and vertical migration.Citation15 The rsmA mutation also resulted in the simultaneous induction of swarming motility, while overexpression of RsmA induced GV production and simultaneously repressed swarming. This may suggest, at least at this regulatory level, that it is an “either/or” decision to float or to swim for Serratia 39006. It is likely that GV production is tightly coupled to the energy status of the cell and given the higher energy requirements of flagella-driven motion,Citation1 we suspect that GV morphogenesis may be favored in conditions where nutrients, energy and oxygen are scarce, thereby effectively acting as intracellular “life preservers” in adverse conditions.
We have shown that Serratia 39006 senses oxygen and BHL (cell density) as environmental parameters and that certain regulatory genes are involved in the control of gas vesicle morphogenesis. However, there is clearly a lot more yet to uncover in terms of the diversity of physiological and environmental control processes involved in gas vesicle development. The co-regulation of gas vesicle assembly with two bioactive secondary metabolites is of particular interest. Colonisation of the air-liquid interface may create new demands for the bacterium - not least the need to defend that new niche against predators or competitors. Carbapenems are broad spectrum antibacterial antibioticsCitation16 and prodigiosins are known to have antibacterial, antifungal and antiprotozoal activity,Citation5 thus these may be useful molecular ammunition for the Serratia strain in defending against bacterial competitors and protozoan grazers.
Despite GVs being discovered over a century ago,Citation1 there is a paucity of basic information on their genetic, developmental and structural biology. Molecular structure studies have mostly focused on the main subunit GvpA, but have been limited to some extent by its intrinsic insolubility. Furthermore, little is known about the other ~10–15 commonly-associated gas vesicle proteins, which include several accessory structural components, potential vesicle nucleation proteins, chaperones and regulatory proteins.Citation1,Citation17,Citation18 Indeed the entire program of structural assembly of the gas vesicle awaits elucidation. Finally, several observations have indicated that GVs may carry out accessory roles in addition to modulating buoyancy. Halobacterial GVs are induced in severe oxidative and H2O2 stress - conditions that could be exacerbated by flotation into an oxygen rich surface. Furthermore, gas vesicle genes have been found in actinomycetesCitation19,Citation20 and functional gas vesicle proteins have been identified in a strain of Bacillus megaterium,Citation21 species conventionally considered to be “non-aquatic.” In Streptomyces coelicolor, GV genes are highly induced in high-salt conditions, indicating a possible role in hyper-osmotic stress.Citation22 These observations may reflect unknown lifestyle aspects of the particular organisms, or they may indicate new and unrecognized roles for GVs in other processes. In summary, we hope that the high genetic tractability of Serratia 39006 will enable faster research progress in GV biology, while illuminating exciting areas of microbial cell biology and macromolecular protein assembly.
Acknowledgments
Work in the Salmond lab is funded by the Biotechnology and Biological Sciences Research Council (BBSRC), UK. J.R. was funded by a Herchel Smith Postdoctoral Fellowship, University of Cambridge. We thank Neil Williamson and other members of the group for helpful discussions.
References
- Walsby AE. Gas vesicles. Microbiol Rev 1994; 58:94 - 144; PMID: 8177173
- Paerl HW, Huisman J. Climate. Blooms like it hot. Science 2008; 320:57 - 8; http://dx.doi.org/10.1126/science.1155398; PMID: 18388279
- Ramsay JP, Williamson NR, Spring DR, Salmond GP. A quorum-sensing molecule acts as a morphogen controlling gas vesicle organelle biogenesis and adaptive flotation in an enterobacterium. Proc Natl Acad Sci USA 2011; 108:14932 - 7; http://dx.doi.org/10.1073/pnas.1109169108; PMID: 21873216
- Parker WL, Rathnum ML, Wells JS Jr., Trejo WH, Principe PA, Sykes RB. SQ 27,860, a simple carbapenem produced by species of Serratia and Erwinia.. J Antibiot (Tokyo) 1982; 35:653 - 60; PMID: 7118721
- Williamson NR, Fineran PC, Gristwood T, Chawrai SR, Leeper FJ, Salmond GP. Anticancer and immunosuppressive properties of bacterial prodiginines. Future Microbiol 2007; 2:605 - 18; http://dx.doi.org/10.2217/17460913.2.6.605; PMID: 18041902
- Williamson NR, Fineran PC, Ogawa W, Woodley LR, Salmond GP. Integrated regulation involving quorum sensing, a two-component system, a GGDEF/EAL domain protein and a post-transcriptional regulator controls swarming and RhlA-dependent surfactant biosynthesis in Serratia.. Environ Microbiol 2008; 10:1202 - 17; http://dx.doi.org/10.1111/j.1462-2920.2007.01536.x; PMID: 18294208
- Beard SJ, Hayes PK, Pfeifer F, Walsby AE. The sequence of the major gas vesicle protein, GvpA, influences the width and strength of halobacterial gas vesicles. FEMS Microbiol Lett 2002; 213:149 - 57; http://dx.doi.org/10.1111/j.1574-6968.2002.tb11299.x; PMID: 12167531
- Dunton PG, Walsby AE. The diameter and critical collapse pressure of gas vesicles in Microcystis are correlated with GvpCs of different length. FEMS Microbiol Lett 2005; 247:37 - 43; http://dx.doi.org/10.1016/j.femsle.2005.04.026; PMID: 15927745
- Bright DI, Walsby AE. The relationship between critical pressure and width of gas vesicles in isolates of Planktothrix rubescens from Lake Zurich. Microbiology 1999; 145:2769 - 75; PMID: 10537198
- West SA, Griffin AS, Gardner A, Diggle SP. Social evolution theory for microorganisms. Nat Rev Microbiol 2006; 4:597 - 607; http://dx.doi.org/10.1038/nrmicro1461; PMID: 16845430
- Thomson NR, Crow MA, McGowan SJ, Cox A, Salmond GP. Biosynthesis of carbapenem antibiotic and prodigiosin pigment in Serratia is under quorum sensing control. Mol Microbiol 2000; 36:539 - 56; http://dx.doi.org/10.1046/j.1365-2958.2000.01872.x; PMID: 10844645
- Walsby AE, Mcallister GK. Buoyancy Regulation by Microcystis in Lake Okaro. New Zeal J Mar Fresh 1987; 21:521 - 4; http://dx.doi.org/10.1080/00288330.1987.9516249
- Jarrell KF, McBride MJ. The surprisingly diverse ways that prokaryotes move. Nat Rev Microbiol 2008; 6:466 - 76; http://dx.doi.org/10.1038/nrmicro1900; PMID: 18461074
- Timmermans J, Van Melderen L. Conditional essentiality of the csrA gene in Escherichia coli.. J Bacteriol 2009; 191:1722 - 4; http://dx.doi.org/10.1128/JB.01573-08; PMID: 19103924
- Walsby AE, Juttner F. The uptake of amino acids by the cyanobacterium Planktothrix rubescens is stimulated by light at low irradiances. FEMS Microbiol Ecol 2006; 58:14 - 22; http://dx.doi.org/10.1111/j.1574-6941.2006.00143.x; PMID: 16958904
- Nicolau DP. Carbapenems: a potent class of antibiotics. Expert Opin Pharmacother 2008; 9:23 - 37; http://dx.doi.org/10.1517/14656566.9.1.23; PMID: 18076336
- Chu LJ, Chen MC, Setter J, Tsai YS, Yang H, Fang X, et al. New structural proteins of Halobacterium salinarum gas vesicle revealed by comparative proteomics analysis. J Proteome Res 2011; 10:1170 - 8; http://dx.doi.org/10.1021/pr1009383; PMID: 21158390
- Offner S, Hofacker A, Wanner G, Pfeifer F. Eight of fourteen gvp genes are sufficient for formation of gas vesicles in halophilic archaea. J Bacteriol 2000; 182:4328 - 36; http://dx.doi.org/10.1128/JB.182.15.4328-4336.2000; PMID: 10894744
- Walsby AE, Dunton PG. Gas vesicles in actinomycetes?. Trends Microbiol 2006; 14:99 - 100; http://dx.doi.org/10.1016/j.tim.2006.01.002; PMID: 16459079
- van Keulen G, Hopwood DA, Dijkhuizen L, Sawers RG. Gas vesicles in actinomycetes: old buoys in novel habitats?. Trends Microbiol 2005; 13:350 - 4; http://dx.doi.org/10.1016/j.tim.2005.06.006; PMID: 15993071
- Li N, Cannon MC. Gas vesicle genes identified in Bacillus megaterium and functional expression in Escherichia coli.. J Bacteriol 1998; 180:2450 - 8; PMID: 9573198
- Lee EJ, Karoonuthaisiri N, Kim HS, Park JH, Cha CJ, Kao CM, et al. A master regulator σB governs osmotic and oxidative response as well as differentiation via a network of sigma factors in Streptomyces coelicolor.. Mol Microbiol 2005; 57:1252 - 64; http://dx.doi.org/10.1111/j.1365-2958.2005.04761.x; PMID: 16101999