Abstract
The small GTPases of the Rho family are key signaling molecules regulating a plethora of biological pathways. They can exert diverse, sometimes opposite, contributions to specific cellular processes explaining why their regulation and their crosstalk must be finely tuned. Several mechanisms driving crosstalk between Rho GTPases have been described in the literature. They implicate proteins regulating their activity or common downstream effectors. Among the proteins regulating Rho GTPases cycling, RhoGDIs were viewed until very recently as passive inhibitors. Here, we will focus on recent data supporting a role for RhoGDIalpha in the crosstalk between RhoGTPases and present our results suggesting that “preferential” RhoGDIalpha-mediated crosstalk takes place between closely related Rho GTPases.
From yeast to higher mammals, numerous vital aspects of cell physiology are driven by Rho GTPases including cytoskeleton organization, cell migration, survival, proliferation or differentiation. The various Rho GTPases can act cooperatively to regulate cell behavior. However, they can also make differential or even opposite contribution to specific cellular processes. Hence, a timely and spatially coordinated regulation of their action is often required to achieve a specific cellular function. This is best illustrated by the spatiotemporal visualization of Cdc42, Rac1 and RhoA signaling by mean of fluorescent resonance energy transfer (FRET)-based biosensors during cell protrusion.Citation1 Such observations highlight the necessary coordination and crosstalk between Rho GTPases to generate efficient cell movement. These signaling molecules function as binary molecular switches shuttling between an inactive GDP-bound state and an active GTP-bound state. The exchange of GDP to GTP is promoted at the plasma membrane by “guanine nucleotide exchange factors” (GEF). The return to an inactive state by hydrolysis of GTP into GDP relies on the catalysis of the intrinsic GTPase activity of RhoGTPases by a “GTPase activating protein” (GAP). Finally, they can be sequestrated in the cytoplasm as an inactive complex with “RhoGDP dissociation inhibitor” (RhoGDI).
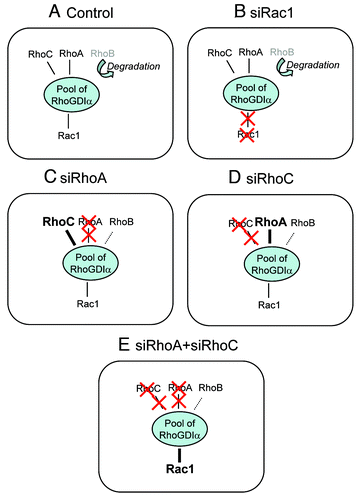
In the recent literature, various mechanisms regulating at different levels the networking between RhoGTPases have been described. These cross-regulations can be mediated via the proteins regulating the activation level of the RhoGTPases or through downstream signaling targets (for review, see Citation2). Among these regulatory proteins, RhoGDIalpha has been recently reported to participate in this crosstalk. RhoGDIs encompass a family of three members with the archetypal RhoGDIalpha being the most abundant and ubiquitous. RhoGDIalpha is a key regulator of Rho GTPases cycling and was further reported to prevent Rho GTPase degradation.Citation3 One of our previous studies underscored the role of RhoGDIalpha in the cross-regulations operating within the Rho subfamily,Citation4 which was recently confirmed and extended by Boulter et al.Citation5 RhoGDIalpha can bind most of the Rho GTPases in a stoechiometric way and its limited availability implies that the individual members of the Rho GTPase family compete for binding.Citation5 Hence, high overexpression of one RhoGTPase will lead to the displacement and degradation of the others without any indication of a preferential order.Citation5 By contrast, our recent studies reporting the regulation of RhoB by RhoA-GDPCitation4 and the RhoGDIalpha-dependent balance between RhoA and RhoCCitation6 showed a cross-regulation limited to the members of the Rho subgroup with Rac1 and Cdc42 expressions and activities remained unaffected.Citation6 Moreover, we also reported that depletion of either Rac1 or Cdc42 did not affect the expression level of RhoA, RhoB or RhoC.Citation4 These observations support the concept that a “preferential” or “ordered” RhoGDI-dependent crosstalk takes place within the Rho GTPase family (). This finely tuned “hierarchy” cannot be seen in extreme conditions such as transient transfection that overwhelm cells with exogenous recombinant Rho proteins, but is only detectable when the RhoGTPases are expressed at levels close to physiological levels. This can be achieved in vitro by using siRNA to reduce specifically the endogenous expression of a single RhoGTPase or by using cells engineered to express any single RhoGTPases under the control of a tightly regulated promoter. According to our new data, there is a preferential competition between the members of the Rho subclass (RhoA, RhoB and RhoC) for interacting with RhoGDIalpha. As a result, modifying the level of any one of these three members inversely and dynamically balances the levels of the two others. The overall interaction between RhoGDIalpha and the members of the Rho subclass considered collectively remains unchanged and prevents any effect on the other RhoGTPases as Rac1 for example. This mechanism is clearly illustrated in models where RhoA and RhoC, the two most abundant members of the Rho subclass, are simultaneously silenced (), which favors the binding of the less abundant RhoB to RhoGDIalpha but also of Rac1. The binding of Rac1 to RhoGDIalpha results in an increased concentration through stabilization and a reduction of its activation likely by preventing its interaction with its GEFs. The molecular mechanisms underlying this “hierarchically ordered” RhoGDI-dependent crosstalk are not yet elucidated but should involve ubiquitous actors since similar regulations were observed in several cell types including prostate and breast adenocarcinoma cell lines and primary skin fibroblasts (Deroanne et al., unpublished results). They may depend on regulatory events able to modify the affinity of RhoGDIalpha toward one specific, or a group of closely related, RhoGTPases. It could be achieved through phosphorylation of various residues in RhoGDIalpha or the RhoGTPases themselves. An alternative mechanism could involve scaffold proteins interacting with RhoGDIalpha able to increase its affinity for RhoA and RhoC, in a similar way as syndecan 4 which together with synectin increases the affinity of RhoGDIalpha for RhoG.Citation7 The formation of such complexes might require the recruitment of RhoGDIalpha in specific sub-cellular compartment(s). Hence, combination of proteomic analyses with classical biochemical methods and live cell imaging should allow further characterization of the crosstalk mediated by RhoGDIalpha and highlight novel pathways and proteins involved in the cross-regulations coordinating spatiotemporally the Rho GTPases signaling.
Figure 1. In control conditions (a), RhoA, RhoC and Rac1 can associate with RhoGDIalpha and RhoB is barely detectable as it is rapidly degraded. The silencing of Rac1 (b) did not affect the interaction of RhoA, RhoB and RhoC with RhoGDIalpha. Upon RhoA silencing (c), RhoC concentration is enhanced through increased binding to RhoGDIalpha and RhoB is stabilized in a RhoGDIalpha-dependent way. The RhoGDIalpha-Rac1 binding is similar to control. Upon RhoC silencing (d), RhoA concentration is enhanced through increased binding to RhoGDIalpha and RhoB is stabilized in a RhoGDIalpha-dependent way. The amount of Rac1 associated to RhoGDIalpha is similar to control. Suppression of the compensation between RhoA and RhoC via the double silencing of RhoA and RhoC enhances the amount of Rac1 associated with RhoGDIalpha (e).
Acknowledgments
This work was partly supported by grants from Belgian FRSM (3.4514.07) to C.F.D. and from the Belspo/Prodex Agency and ESA to B.V.N. T.T.G.H and A.S. were fellows of the Belgian F.N.R.S (FRIA and Televie). A.C.C. is a Senior Research Associate and C.F.D is a Research Associate both at the Belgian F.R.S.-FNRS.
References
- Machacek M, Hodgson L, Welch C, Elliott H, Pertz O, Nalbant P, et al. Coordination of Rho GTPase activities during cell protrusion. Nature 2009; 461:99 - 103; http://dx.doi.org/10.1038/nature08242; PMID: 19693013
- Guilluy C, Garcia-Mata R, Burridge K. Rho protein crosstalk: another social network?. Trends Cell Biol 2011; In press http://dx.doi.org/10.1016/j.tcb.2011.08.002; PMID: 21924908
- Bielek H, Anselmo A, Dermardirossian C. Morphological and proliferative abnormalities in renal mesangial cells lacking RhoGDI. Cell Signal 2009; 21:1974 - 83; http://dx.doi.org/10.1016/j.cellsig.2009.09.008; PMID: 19765647
- Ho TT, Merajver SD, Lapière CM, Nusgens BV, Deroanne CF. RhoA-GDP regulates RhoB protein stability. Potential involvement of RhoGDIalpha. J Biol Chem 2008; 283:21588 - 98; http://dx.doi.org/10.1074/jbc.M710033200; PMID: 18524772
- Boulter E, Garcia-Mata R, Guilluy C, Dubash A, Rossi G, Brennwald PJ, et al. Regulation of Rho GTPase crosstalk, degradation and activity by RhoGDI1. Nat Cell Biol 2010; 12:477 - 83; http://dx.doi.org/10.1038/ncb2049; PMID: 20400958
- Giang Ho TT, Stultiens A, Dubail J, Lapière CM, Nusgens BV, Colige AC, et al. RhoGDIα-dependent balance between RhoA and RhoC is a key regulator of cancer cell tumorigenesis. Mol Biol Cell 2011; 22:3263 - 75; http://dx.doi.org/10.1091/mbc.E11-01-0020; PMID: 21757538
- Elfenbein A, Rhodes JM, Meller J, Schwartz MA, Matsuda M, Simons M. Suppression of RhoG activity is mediated by a syndecan 4-synectin-RhoGDI1 complex and is reversed by PKCalpha in a Rac1 activation pathway. J Cell Biol 2009; 186:75 - 83; http://dx.doi.org/10.1083/jcb.200810179; PMID: 19581409