Abstract
Since our initial demonstrations that hydrogen sulfide (H2S) may function as a neuromodulator in the brain and a smooth muscle relaxant in the vascular system, accumulating evidence shows that H2S may function as a signaling molecule. We and others also found that H2S has a cytoprotective effect. Because H2S is well-known toxic gas, a cytoprotective role has been overlooked. H2S protects neurons from oxidative stress. It also protects cardiac muscle from ischemia-reperfusion injury. The finding led to the application of H2S to the bypass surgery patients in Phase II clinical trial. Cystathionine β–synthase (CBS) and cystathionine γ–lyase (CSE) are well known as H2S-producing enzymes. We recently demonstrated that the other H2S-producing enzyme, 3-mercaptopyruvate sulfurtransferase (3MST) along with cysteine aminotransferase (CAT) is localized to neurons in the brain and to the vascular endothelium. However, the regulation of H2S production by 3MST/CAT pathway had not been well understood. The present study shows that H2S production by 3MST/CAT pathway is regulated by Ca2+ and that H2S protects retinal photoreceptor cells from light induced degeneration by suppressing excessive Ca2+ influx caused by intense light.
We demonstrated that CBS is expressed in the brain and can produce H2S, which facilitates the induction of hippocampal long-term potentiation (LTP), a synaptic model of memory, by enhancing the activity of NMDA receptors.Citation1 H2S also induces Ca2+ influx and Ca2+ waves in astrocytes.Citation2,Citation3 Another H2S-producing enzyme, CSE, was found in the thoracic aorta, the ileum and the portal vein and H2S relaxes these tissues.Citation4 Based on these observations we proposed that H2S may function as a neuromodulator and a smooth muscle relaxant. Subsequently, H2S was found to activate ATP-dependent K+ channels to relax vascular smooth muscle.Citation5 In addition to the function as a signaling molecule, H2S also has a role as a cytoprotectant.Citation6-Citation10 It protects neurons from oxidative stress by reinstating the levels of glutathione, an intracellular major antioxidant.Citation6-Citation8 It also protects cardiac muscle from ischemia-reperfusion injury by preserving the mitochondrial function.Citation10
In the brain CBS is localized to astrocytes,Citation11,Citation12 a type of glia, while 3MST is localized to neurons.Citation13 3MST and CAT localized to vascular endothelium also produce H2S that may regulate vascular tone.Citation14 3MST produces H2S from 3-mercaptopyruvate, which is produced by CAT from cysteine and α–ketoglutarate. H2S production by 3MST/CAT pathway requires a reducing substance, such as dithiothreitol (DTT). However, the corresponding endogenous reducing substance has not been identified. We recently demonstrated that thioredoxin and dihydrolipoic acid (DHLA) are endogenous reducing substances for 3MST to produce H2S.Citation15
3MST along with CAT is also localized to retinal neurons, and H2S production by the enzymes is regulated by Ca2+.Citation16 In the absence of Ca2+ the production is the maximum and is decreased by Ca2+ in a concentration-dependent manner. There is no change in the activity of 3MST/CAT pathway to produce H2S in the presence or absence of calmodulin or a calmodulin inhibitor, W-7, suggesting that calmodulin is not involved in the regulation on the pathway by Ca2+ ().Citation16
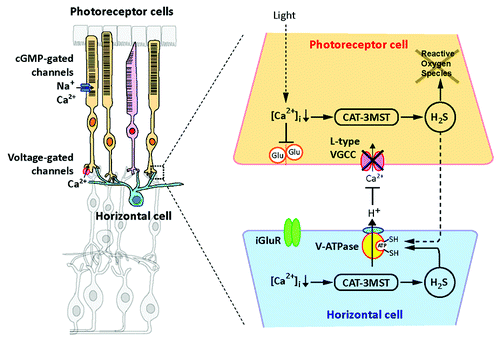
The center-surround organization is one of the most important characteristics in the retinal neurons. The negative feedback from horizontal cells to photoreceptor cells plays a key role in the center-surround organization. When retinal photoreceptor cells are exposed to light, the intracellular concentrations of Ca2+ are decreased to 10 nM that activates 3MST/CAT pathway to produce H2S. In darkness Ca2+ concentrations are increased to 600 nM that cause the cessation of H2S production. H2S, in turn, suppresses voltage-gated L-type Ca2+ channels (VGCC) in photoreceptor cells by activating vacuolar-type H+-ATPase (V-ATPase) in horizontal cells, leading to maintaining intracellular Ca2+ in photoreceptor cells in low levels ().Citation16
The retina is susceptible to oxidative stress because of its high consumption of oxygen and daily exposure to light. Excessive light exposure leads to photoreceptor degeneration whose death is an irreversible injury caused by reactive oxygen species and elevated intracellular concentrations of Ca2+. The regulation of Ca2+ by endogenous H2S may fail by the excessive levels of light, and the photoreceptor cell degeneration occurs. Even under such conditions the administration of a donor of H2S suppresses photoreceptor degeneration. The increased number of TUNEL- and 8-hydroxy-2’-deoxyguanosine positive cells by intense light was decreased by administration of H2S.Citation16 The enhancement of 3MST/CAT pathway or the administration of H2S may have clinical benefit for diseases with retinal cell degeneration.
Figure 1. When retinal photoreceptor cells are exposed to light, cGMP-gated channels are closed and the cell membrane is hyperpolarized. The intracellular concentrations of Ca2+ in photoreceptor cells are decreased to approximately 10 nM, which activates 3MST/CAT to produce H2S. H2S activates vacuolar-type H+-ATPase in horizontal cells to released H+ that suppresses the activity of voltage gated Ca2+ channels in photoreceptor cells. By this mechanism H2S maintains intracellular Ca2+ in low levels. Excessive light exposure leads to photoreceptor degeneration caused by reactive oxygen species and elevated intracellular concentrations of Ca2+. The regulation of Ca2+ by endogenous H2S may fail by the excessive levels of light, and the photoreceptor cell degeneration occurs. Even under such conditions the enhancement of 3MST/CAT pathway or the administration of H2S may have clinical benefit for diseases with retinal cell degeneration.
| Abbreviations: | ||
| H2S | = | hydrogen sulfide |
| CBS | = | cystathionine β–synthase |
| CSE | = | cystathionine γ–lyase |
| 3MST | = | 3-mercaptopyruvate sulfurtransferase |
| CAT | = | cysteine aminotransferase |
| LTP | = | long-term potentiation |
| DTT | = | dithiothreitol |
| DHLA | = | dihydrolipoic acid |
| VGCC | = | voltage-gated Ca2+ |
| V-ATPase | = | vacuolar-type H+-ATPase |
Acknowledgments
This work was supported by a grant from National Institute of Neuroscience and by KAKENHI (23659089) from Grant-in-Aid for Challenging Exploratory Research to H.K., by KAKENHI (23790316) from Grant-in-Aid for Young Scientists (B) to Y.M.
References
- Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 1996; 16:1066 - 71; PMID: 8558235
- Nagai Y, Tsugane M, Oka J, Kimura H. Hydrogen sulfide induces calcium waves in astrocytes. FASEB J 2004; 18:557 - 9; PMID: 14734631
- Tsugane M, Nagai Y, Kimura Y, Oka J-I, Kimura H. Differentiated astrocytes acquire sensitivity to hydrogen sulfide that is diminished by the transformation into reactive astrocytes. Antioxid Redox Signal 2007; 9:257 - 69; http://dx.doi.org/10.1089/ars.2007.9.257; PMID: 17115938
- Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 1997; 237:527 - 31; http://dx.doi.org/10.1006/bbrc.1997.6878; PMID: 9299397
- Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 2001; 20:6008 - 16; http://dx.doi.org/10.1093/emboj/20.21.6008; PMID: 11689441
- Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 2004; 18:1165 - 7; PMID: 15155563
- Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 2006; 8:661 - 70; http://dx.doi.org/10.1089/ars.2006.8.661; PMID: 16677109
- Kimura Y, Goto Y-I, Kimura H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid Redox Signal 2010; 12:1 - 13; http://dx.doi.org/10.1089/ars.2008.2282; PMID: 19852698
- Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, Cheung NS, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite ‘scavenger’?. J Neurochem 2004; 90:765 - 8; http://dx.doi.org/10.1111/j.1471-4159.2004.02617.x; PMID: 15255956
- Elrod JW, Calvert JW, Morrison J, Doeller JE, Kraus DW, Tao L, et al. Hydrogen sulfide attenuates myocardial ischemia-reperfusion injury by preservation of mitochondrial function. Proc Natl Acad Sci U S A 2007; 104:15560 - 5; http://dx.doi.org/10.1073/pnas.0705891104; PMID: 17878306
- Enokido Y, Suzuki E, Iwasawa K, Namekata K, Okazawa H, Kimura H. Cystathionine β-synthase, a key enzyme for homocysteine metabolism, is preferentially expressed in the radial glia/astrocyte lineage of developing mouse CNS. FASEB J 2005; 19:1854 - 6; PMID: 16160063
- Ichinohe A, Kanaumi T, Takashima S, Enokido Y, Nagai Y, Kimura H. Cystathionine β-synthase is enriched in the brains of Down’s patients. Biochem Biophys Res Commun 2005; 338:1547 - 50; http://dx.doi.org/10.1016/j.bbrc.2005.10.118; PMID: 16274669
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, Ishii K, et al. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 2009; 11:703 - 14; http://dx.doi.org/10.1089/ars.2008.2253; PMID: 18855522
- Shibuya N, Mikami Y, Kimura Y, Nagahara N, Kimura H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J Biochem 2009; 146:623 - 6; http://dx.doi.org/10.1093/jb/mvp111; PMID: 19605461
- Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sulfurtransferase to produce hydrogen sulfide. Biochem J 2011; 439:479 - 85; http://dx.doi.org/10.1042/BJ20110841; PMID: 21732914
- Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, Kimura H. Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem 2011; 286:39379 - 86; http://dx.doi.org/10.1074/jbc.M111.298208; PMID: 21937432