Abstract
Neurons become terminally differentiated (TD) post-mitotic cells very early during development yet they may remain alive and functional for decades. TD neurons preserve the molecular machinery necessary for DNA synthesis that may be reactivated by different stimuli but they never complete a successful mitosis. The non-reversible nature of the post-mitotic state in neurons suggests a non-genetic basis for it since no set of mutations has been able to revert it. Comparative studies of the nuclear higher-order structure in neurons and cells with proliferating potential suggest that the non-reversible nature of the post-mitotic state in neurons has a structural basis in the stability of the nuclear higher-order structure.
Background
A mature, terminally differentiated (TD) cell no longer able to undergo mitosis is defined as post-mitotic. Traditionally TD cells which are stably post-mitotic are considered to be permanently outside form the cell cycle and yet there is ample evidence that typical TD cells such as neurons and cardiomyocytes express the molecular machinery necessary for DNA synthesis that may be reactivated either by cellular stressors or experimental manipulation.Citation1,Citation2 In mammals cortical neurons become post-mitotic quite early during development, after leaving the germinal centers in the ventricular zoneCitation3 but nevertheless they may remain alive and functional in the long-term (actually decades in the case of humans) without any change in their post-mitotic condition. Indeed, there is compelling evidence that in humans no new neurons are added to the neocortex after birth.Citation4 Moreover, the resistance of TD neurons to further cell division is so absolute that so far no brain tumors derived from mature neurons have occurred spontaneously or been induced by carcinogens in the adult cortex.Citation5 The old hypothesis that brain tumors arise from dedifferentiation of mature brain cells in response to genetic mutations was assumed facing the evidence that the normal postnatal brain has no proliferating potential. Yet the discovery in the adult brain of neural stem cells able to generate all the required specialized cell types: neurons, astrocytes and oligodendrocytesCitation6 suggests that such cells are the target of the transformation events leading to a brain tumor.Citation7 In the case of TD muscle cells blocking the activity of inhibitors of cyclin-dependent kinases leads to reentry into the cell cycle of such TD cells. Yet successful completion of the new cell cycle has not been observed as the reactivated cells cannot complete proper DNA replication and they undergo cell death or arrest indefinitely in the G2 phase of the first cell cycle.Citation8 From observations like these it has been suggested that the post-mitotic state is an active state mediated by specific gene productsCitation2 but that suggestion goes against wider evidence that any cellular process or state that depends on the action of specific gene products acting in trans can be either blocked or reverted by mutations (either spontaneous or induced) in the corresponding genes but this has never been observed in the case of post-mitotic neurons. Moreover, in the case of TD neurons reentry into the cell cycle either induced by cellular stress or experimental manipulation is always lethal.Citation1,Citation9-Citation11 This cell cycle related neuronal death (CRND) has been linked to pathological neurodegenerative processesCitation7,Citation12 and there is evidence that CDK5, a nontraditional cyclin-dependent kinase very active in TD neurons, is a potent suppressor of the cell cycle in neurons thus playing a critical neuroprotective role by avoiding CRND.Citation13,Citation14
DNA and Structural Stress
The chromosomes of the metazoans genomes are constituted by very long continuous DNA molecules. The general molecular structure of DNA is characterized by a rather rigid double backbone made by the high-energy, phosphodiester bonds between the deoxyribose sugar moieties of the many constituting nucleotides, such a double backbone defines anti-parallel strands linked by quasi-statistical, low-energy, hydrogen bonds between the nitrogenous bases of the corresponding nucleotides in such anti-parallel strands, this results in the formation of a molecular double helix subjected to important structural stress along the molecule’s length. The structural stress of DNA along its axis may be dissipated by breaking the hydrogen bonds between both strands, yet by looping and supercoiling along its axis DNA can dissipate the stress without compromising its structural integrity.Citation15 Thus forming DNA loops along a chromosome is a natural solution for dissipating structural stress along the DNA molecule,Citation16-Citation19 but this looping can be stabilized by the interaction of DNA with some other material within the nucleus.
Nuclear Higher-Order Structure
Currently there is important evidence for the existence in metazoan cells of a nucleoskeleton involved in the organization of the genome.Citation20 Indeed, the nucleus is crowded with proteins many of which perform a structural role. The nuclear matrix (NM) has been operationally defined as a high-salt insoluble nuclear compartment constituted by a sort of dynamic fibrogranular network which after extraction retains the shape and some morphological features of the nucleus.Citation21-Citation23 The exact composition of the nuclear matrix (NM) is a matter of debate as some four hundred proteins have been associated with such a compartment and there is evidence that some NM proteins may be common to many cell types while others may be cell-type specific.Citation24-Citation27 Thus in the interphase nucleus of metazoan cells DNA is organized in supercoiled, topologically constrained loops anchored to the NM.Citation28,Citation29 DNA is attached or addressed to the NM by non-coding sequences known as matrix associated or matrix attachment regions (MARs). So far in mammals there are no specific consensus sequences for a priori defining a MAR although most well-characterized MARs are relatively rich in A-T and repetitive sequences and map to regions where the DNA is intrinsically curved or kinked and has a propensity for base unpairing.Citation30 In situ MARs have been operationally classified into structural-constitutive, resistant to high-salt extraction, and functional-facultative, non-resistant to high-salt extraction.Citation31,Citation32 Therefore the resulting DNA loops can be also classified into structural and functional.Citation30,Citation33,Citation34 The high-salt resistant MARs attaching the structural DNA loops to the NM are also known as loop anchorage regions or LARs.Citation31 When multiple copies of a specific MAR are present these are used in a selective fashion indicating adaptability of the MAR sequence to serve as anchor only when needed.Citation35 A corollary is that not all potential MARs present in DNA are actually attached to the NM. The interactions between DNA and the NM define a higher-order structure (NHOS) in the interphase nucleus.Citation36,Citation37
Common Properties of Senescent and Post-Mitotic Cells
Classical studies have shown that normal cells with proliferating potential lose such a potential in a stochastic and non-reversible fashion independently of their previous number of cell divisions thus achieving a state of replicative senescence.Citation38-Citation40 Such a stochastic replicative senescence (SRS) is not the consequence of cellular stress thus different of premature RS or STASIS which depends on the action of specific gene products and so can be reverted or rescued by specific mutations.Citation41 Also such a SRS is independent of telomere attrition since it has been observed in cells of rodents that possess very lengthy telomeres that do not shorten with each cell division as telomerase is continuously active in rodent somatic cells.Citation42,Citation43 For example, in the rat liver the hepatocytes are usually quiescent yet they preserve a remarkable proliferating potential so that a 70% partial hepatectomy leads to complete regeneration of the liver mass within seven days after surgery.Citation44 In young adult rats some 95% of the remaining hepatocytes re-enter the cell cycle in order to achieve liver regeneration. However in healthy but older animals the fraction of remaining hepatocytes able to re-enter the cell cycle after liver injury is reduced to < 70%.Citation45,Citation46 This spontaneous loss of proliferating potential as a function of age has been linked to the terminal differentiation of the hepatocytes.Citation47 Indeed, the SRS of aged hepatocytes observed in vivo is a de facto long-term, post-mitotic state.
A common characteristic of tissue enriched with post-mitotic cells is the presence of polyploid cells. For example, in normal adult human brain hyperploidy is commonly detectedCitation48,Citation49 and the number of tetraploid neurons has been estimated at 6–12%.Citation50 Such neurons do not express markers of the cell cycle indicating that they did not re-enter the cell cycle and so could be considered as a static population of tetraploid cells resulting from aborted nuclear disassembly (karyokinesis) and mitosis in the progenitor cells.Citation51 Interestingly, in rats post-natal hepatic growth occurs by rapid cell proliferation followed by cell hypertrophy that is marked by the appearance of polyploid hepatocytes.Citation52 Appearance of nuclear polyploidy is a critical event in hepatocyte differentiation and is associated with cessation of mitotic activity as well as terminal differentiation and senescence.Citation47,Citation53 For example, in rats at post-natal day 21 93% of the hepatocytes are diploid and only 2.5% tetraploid while at 18 mo of age 39% of the hepatocytes are diploid and 41% are tetraploid.Citation54 Yet after partial hepatectomy there is a depletion of diploid cells with an associated relative increase in the proportion of polyploid cells in the regenerated liver and the fraction of polyploid cells further increases after repeated partial hepatectomies.Citation55,Citation56 These facts suggest that such polyploid cells result from the ever increasing fraction of cells that, despite their ability for performing DNA synthesis, stochastically lose their proliferating potential a phenomenon that correlates with their impaired capacity for sister-chromatid segregation (SCS), karyokinesis and mitosis.
A Common Basis for SRS and the Post-Mitotic State
In primary rat hepatocytes the DNA-NM interactions are very labile in the early postnatal period and DNA-loop size is heterogeneous but large on average. Yet such interactions increase in strength and number as a function of time so that DNA loops become shorter on average and more homogeneous in size as a function of age and this correlates with the loss of proliferating potential.Citation57 Based on such evidence the hypothesis was put forward that SRS has a structural, non-genetic basis resulting from thermodynamic constraints acting upon DNA that lead to an ever increasing number of DNA-NM interactions in order to dissipate DNA structural stress.Citation36 Therefore DNA loops become shorter, more numerous and more homogeneous in size as a function of time. Hence the NHOS defined by the DNA-NM interactions becomes more stable on average with age (time). Such a stable nuclear structure requires high activation energy for being destabilized in order to be permissive for SCS and karyokinesis. Thus after passing a certain threshold the stability of the NHOS may become an insurmountable energy barrier for proper karyokinesis and the corresponding cell becomes stably post-mitotic. A prediction of this hypothesis is that the NHOS of early post-mitotic cells should be at least as stable as that observed in aged hepatocytes that have spontaneously lost their proliferating potential. This prediction was confirmed by comparing the NHOS of cortical neurons from baby and aged rats (post-natal days 7 and 540) with that of aged hepatocytes (P540). The results indicated that even in neurons from baby rats (P7) the NHOS is already very stable up to a higher degree than that observed in aged hepatocytes.Citation37 Considering the suggestion that the formation of structural DNA loops by interaction with the NM obeys thermodynamic and structural constraints, a further prediction of the hypothesis was that the NHOS should carry on stabilizing as a function of time even in an already post-mitotic nucleus, provided that there is any remaining DNA-structural stress to be dissipated.Citation36 This means that the NHOS evolves toward maximal stability in time independently of the functional needs of the nucleus. This prediction was confirmed by comparing the NHOS in cortical neurons from P0, P7, P80 and P540 rats. The results indicated that the trend toward further stabilization of the NHOS in neurons continues throughout post-natal life and that this phenomenon occurs in absence of overt changes in the post-mitotic state and transcriptional activity of neurons, suggesting that it is independent of functional constraints in the nucleus.Citation58
NHOS and Thermodynamics
From the structural perspective, the topological organization of the NHOS based on selective use of a limited set of potential MARs, as seen in nuclei from hepatocytes of newborn and baby animalsCitation57 is highly asymmetrical and the natural trend in physical systems is toward reducing the asymmetries in such a way that the system evolves in time so as to become more symmetrical.Citation59,Citation60 A topological configuration in which most potential MARs are actually bound to the NM, thus resulting in shorter and more stable DNA loops, is a more symmetrical structural attractor. According to the current notion entropy is not a measure of disorder or chaos, but of energy diffusion, dissipation or dispersion in a final state compared with an initial state,Citation61 thus a highly-stable DNA-loop configuration satisfies the second law of thermodynamics since the structural stress along the DNA molecule is more evenly dispersed within the nuclear volume by increasing the number of DNA-NM interactions (thus increasing, in terms of molecular thermodynamics, the occupancy of more microstates in phase space).
Nuclear Tensegrity
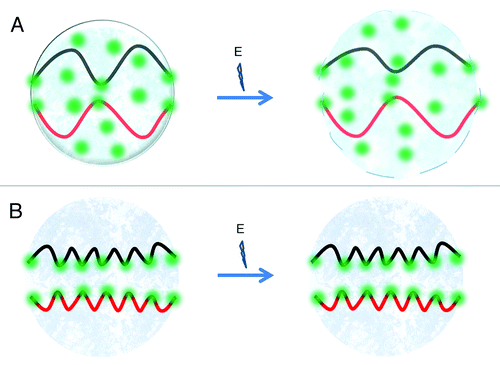
There is ample evidence that the cell is a high-wired system able to transduce mechanical information. Indeed, cells within solid tissues are part of a continuum system of mechano-transduction that couples the extracellular matrix, with the cytoskeleton and the cell nucleus.Citation62 Thus the cell can be modeled as a vector field in which the mechanically linked cytoskeleton-NM-DNA may act as transducers of mechanical information.Citation63 The concept of tensegrity defines structures composed by continuous tension elements and discontinuous compression elements, in such systems the role of the compression elements is minimized and the force is distributed among tension elements that can be slender and lightweight.Citation64 There is plenty of experimental evidence that both cell and tissue tensegrity are a biological fact.Citation65,Citation66 Thus, a large number of DNA-NM interactions may create a structural complex based on tensegrity, in which discontinuous compression elements (proteins) and tensors (DNA) interact for creating a highly stable overall structure (). There is evidence that telomeres are attached to the NMCitation67 while elements of the NM participate in the formation of the chromosome scaffold that constitutes the structural core of mitotic chromosomes.Citation68-Citation70 However, if the number of stable interactions between DNA and the NM increases over time they could reach a point in which the energy input necessary for SCS and destabilization (disassembly) of the cell nucleus is beyond the capacity of the cell (). This threshold of structural stability may determine the long-term post-mitotic state that is independent of the action of soluble factors acting in trans. Since this process obeys thermodynamic constraints it must follow a stochastic behavior that nevertheless increases its probability as a function of time.
Figure 1. Drawing schematizing the interaction between interphase chromosomes and the nuclear matrix (NM) (A) In cells with proliferating potential interphase chromosomes (only two are shown as black and red fibers) are attached the peripheral NM but to very few elements of the internal NM (green spots) thus forming a relatively limited number of rather large DNA loops. In this configuration chromosome DNA preserves significant structural stress and so it has a high dynamic potential. Input of biochemical energy may easily destabilize the nuclear higher order structure, defined by the DNA-NM interactions, leading to karyokinesis and mitosis. (B) In TD cells the interphase chromosomes are organized into a large number of shorter DNA loops attached to many elements of the internal NM, this organization dissipates most DNA structural stress and so DNA loses most of its dynamic potential becoming and integral component of a very stable structural system (perhaps of the tensegrity type) constituted by a large number of DNA-NM interactions that cannot be destabilized by the available input of biochemical energy. Under such a configuration the nucleus cannot be disassembled and so no mitosis may ensue. Thus in order to preserve a proliferating potential the cells cannot dissipate DNA structural stress beyond a certain threshold without becoming stably post-mitotic.
A Stable NHOS as a Barrier for Efficient DNA Synthesis
Early death is observed in neurons forced to re-enter the cell cycleCitation13 and neuronal cell cycle activity has been observed early in several diseases that course with neurodegeneration.Citation7 Other reactivated post-mitotic TD cells such as myotubes die very quickly, from apotosis, after re-entry into the cell cycle. In this case the apoptotic process is triggered by significant DNA damage resulting from the attempted DNA replication and those few myotubes that proceed to mitosis show aberrant mitotic spindles and other serious mitotic anomalies before dying.Citation8 There is important evidence that structural DNA loops correspond to the actual replicons in vivo and that replication occurs in macromolecular complexes organized upon the NM.Citation31,Citation32,Citation71,Citation72 Thus, considering that highly stable physical systems have a much reduced dynamic potential, resulting from intrinsically high activation-energy barriers, it is likely that forced DNA replication in post-mitotic cells having a highly stable NHOS leads to severe replicon damage and cellular death, as it has been observed.Citation8 The fact that the non-canonical cyclin-dependent kinase CDK5 is highly active in neurons so as to inhibit their possible re-entry into the cell cycle that may lead to replicative stress and cellular deathCitation13,Citation14 ties in with the notion that the structural, non-reversible post-mitotic state in neurons requires active safeguards against their possible re-entry into the cell cycle otherwise the neurons will die. Indeed, the homozygous null mutation of CDK5 is embryonic lethal and the aborted embryos display many neuronal abnormalities.Citation73,Citation74
Why Neurons Become Early Post-Mitotic
Why some cells reach the post-mitotic state rather early in life (neurons) while others may have not reached that state even at the end of the usual life expectancy in the wild (hepatocytes). A possible answer to this puzzle is the presence of tissue-specific NM proteins that may increase or facilitate the DNA-NM interactions. For example, NeuN/Fox3 is an abundant neuron-specific protein that is an intrinsic component of the neuronal NM and shows significant DNA-binding properties.Citation27,Citation75 So far very few specific MAR-binding proteins have been identifiedCitation23,Citation30 yet the structural DNA-NM interactions occur on a grand scale despite the fact that there are no MAR consensus sequences, implying that such interactions are the result of indirect readouts between DNA and NM proteins.Citation76 Such protein-DNA indirect readouts depend on local DNA shape and overall DNA mechanical properties such as curvature, helical twist, bending and torsional flexibilities.Citation15,Citation33,Citation76 In blastomeres the genome is organized into a large number of short DNA loops that constitute highly dynamic repliconsCitation77 but such early embryonic cells lack lamins A/C which are important components of the NM of differentiated cellsCitation78 thus the observed maturation of the NM composition during development leads to stabilization of the DNA-NM interactions.Citation57 On the other hand, during embryogenesis there are changes in the rate or timing of development of some cell lineages in the body relative to others (heterochrony), so that different cell lineages develop at different rates.Citation79 Mechano-transduction during tissue morphogenesis may induce changes in the differentiation state of cells and such a modification of the differentiation state may also impinge on the potential morphogenetic trajectory by limiting the repertory of changes in cellular size and shape. Heterochrony may alter the distribution of probabilities of stochastic events such as the rate of actualization of the DNA-NM interactions, thus some cell types such as neurons may on average reach terminal differentiation and became post-mitotic earlier than other cell types, depending on their morphogenetic trajectory.
Acknowledgments
This work was sponsored by CONACYT-México grant 48447-Q (25506). I thank MB Evangelina Silva-Santiago for drawing the figure.
References
- Park DS, Obeidat A, Giovanni A, Greene LA. Cell cycle regulators in neuronal death evoked by excitotoxic stress: implications for neurodegeneration and its treatment. Neurobiol Aging 2000; 21:771 - 81; http://dx.doi.org/10.1016/S0197-4580(00)00220-7; PMID: 11124421
- Pajalunga D, Mazzola A, Salzano AM, Biferi MG, De Luca G, Crescenzi M. Critical requirement for cell cycle inhibitors in sustaining nonproliferative states. J Cell Biol 2007; 176:807 - 18; http://dx.doi.org/10.1083/jcb.200608109; PMID: 17353358
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science 1974; 183:425 - 7; http://dx.doi.org/10.1126/science.183.4123.425; PMID: 4203022
- Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Björk-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci U S A 2006; 103:12564 - 8; http://dx.doi.org/10.1073/pnas.0605177103; PMID: 16901981
- Rakic P. Neuroscience. No more cortical neurons for you. Science 2006; 313:928 - 9; http://dx.doi.org/10.1126/science.1131713; PMID: 16917050
- Alvarez-Buylla A, Lim DA. For the long run: maintaining germinal niches in the adult brain. Neuron 2004; 41:683 - 6; http://dx.doi.org/10.1016/S0896-6273(04)00111-4; PMID: 15003168
- Demir O, Singh S, Klimaschewski L, Kurnaz IA. From birth till death: neurogenesis, cell cycle, and neurodegeneration. Anat Rec (Hoboken) 2009; 292:1953 - 61; http://dx.doi.org/10.1002/ar.20980; PMID: 19943348
- Pajalunga D, Puggioni EMR, Mazzola A, Leva V, Montecucco A, Crescenzi M. DNA replication is intrinsically hindered in terminally differentiated myotubes. PLoS One 2010; 5:e11559; http://dx.doi.org/10.1371/journal.pone.0011559; PMID: 20644635
- Feddersen RM, Ehlenfeldt R, Yunis WS, Clark HB, Orr HT. Disrupted cerebellar cortical development and progressive degeneration of Purkinje cells in SV40 T antigen transgenic mice. Neuron 1992; 9:955 - 66; http://dx.doi.org/10.1016/0896-6273(92)90247-B; PMID: 1419002
- al-Ubaidi MR, Hollyfield JG, Overbeek PA, Baehr W. Photoreceptor degeneration induced by the expression of simian virus 40 large tumor antigen in the retina of transgenic mice. Proc Natl Acad Sci U S A 1992; 89:1194 - 8; http://dx.doi.org/10.1073/pnas.89.4.1194; PMID: 1311085
- Appert-Collin A, Hugel B, Levy R, Niederhoffer N, Coupin G, Lombard Y, et al. Cyclin dependent kinase inhibitors prevent apoptosis of postmitotic mouse motoneurons. Life Sci 2006; 79:484 - 90; http://dx.doi.org/10.1016/j.lfs.2006.01.032; PMID: 16530228
- Zekanowski C, Wojda U. Aneuploidy, chromosomal missegregation, and cell cycle reentry in Alzheimer’s disease. Acta Neurobiol Exp (Wars) 2009; 69:232 - 53; PMID: 19593337
- Zhang J, Herrup K. Cdk5 and the non-catalytic arrest of the neuronal cell cycle. Cell Cycle 2008; 7:3487 - 90; http://dx.doi.org/10.4161/cc.7.22.7045; PMID: 19001851
- Zhang J, Li H, Yabut O, Fitzpatrick H, D’Arcangelo G, Herrup K. Cdk5 suppresses the neuronal cell cycle by disrupting the E2F1-DP1 complex. J Neurosci 2010; 30:5219 - 28; http://dx.doi.org/10.1523/JNEUROSCI.5628-09.2010; PMID: 20392944
- Calladine CR, Drew HR, Luisi BF, Travers AA. Understanding DNA, 3rd ed. London; Elsevier-Academic Press 2004;116-138.
- Ringrose L, Chabanis S, Angrand PO, Woodroofe C, Stewart AF. Quantitative comparison of DNA looping in vitro and in vivo: chromatin increases effective DNA flexibility at short distances. EMBO J 1999; 18:6630 - 41; http://dx.doi.org/10.1093/emboj/18.23.6630; PMID: 10581237
- Yan J, Kawamura R, Marko JF. Statistics of loop formation along double helix DNAs. Phys Rev E Stat Nonlin Soft Matter Phys 2005; 71:061905; http://dx.doi.org/10.1103/PhysRevE.71.061905; PMID: 16089763
- Diesinger PM, Kunkel S, Langowski J, Heermann DW. Histone depletion facilitates chromatin loops on the kilobasepair scale. Biophys J 2010; 99:2995 - 3001; http://dx.doi.org/10.1016/j.bpj.2010.08.039; PMID: 21044597
- Rosa A, Becker NB, Everaers R. Looping probabilities in model interphase chromosomes. Biophys J 2010; 98:2410 - 9; http://dx.doi.org/10.1016/j.bpj.2010.01.054; PMID: 20513384
- Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic ‘network of networks’. Nat Rev Mol Cell Biol 2011; 12:695 - 708; http://dx.doi.org/10.1038/nrm3207; PMID: 21971041
- Razin SV. Nuclear matrix and the spatial organization of chromosomal DNA domains. Austin, TX: RG Landes Co 1997; 1-23.
- Nickerson JA. Experimental observations of a nuclear matrix. J Cell Sci 2001; 114:463 - 74; PMID: 11171316
- Tsutsui KM, Sano K, Tsutsui K. Dynamic view of the nuclear matrix. Acta Med Okayama 2005; 59:113 - 20; PMID: 16155636
- Fey EG, Penman S. Nuclear matrix proteins reflect cell type of origin in cultured human cells. Proc Natl Acad Sci U S A 1988; 85:121 - 5; http://dx.doi.org/10.1073/pnas.85.1.121; PMID: 3277168
- Stuurman N, Meijne AML, van der Pol AJ, de Jong L, van Driel R, van Renswoude J. The nuclear matrix from cells of different origin. Evidence for a common set of matrix proteins. J Biol Chem 1990; 265:5460 - 5; PMID: 2180926
- Mika S, Rost B. NMPdb: database of nuclear matrix proteins. Nucleic Acids Res 2005; 33:Database issue D160 - 3; http://dx.doi.org/10.1093/nar/gki132; PMID: 15608168
- Dent MA, Segura-Anaya E, Alva-Medina J, Aranda-Anzaldo A. NeuN/Fox-3 is an intrinsic component of the neuronal nuclear matrix. FEBS Lett 2010; 584:2767 - 71; http://dx.doi.org/10.1016/j.febslet.2010.04.073; PMID: 20452351
- Cook PR, Brazell IA, Jost E. Characterization of nuclear structures containing superhelical DNA. J Cell Sci 1976; 22:303 - 24; PMID: 1002771
- Roti-Roti JL, Wright WD, Taylor YC. DNA loop structure and radiation response. Adv Radiat Biol 1993; 17:227 - 59
- Ottaviani D, Lever E, Takousis P, Sheer D. Anchoring the genome. Genome Biol 2008; 9:201; http://dx.doi.org/10.1186/gb-2008-9-1-201; PMID: 18226181
- Razin SV. The nuclear matrix and chromosomal DNA loops: is their any correlation between partitioning of the genome into loops and functional domains?. Cell Mol Biol Lett 2001; 6:59 - 69; PMID: 11544631
- Maya-Mendoza A, Herńndez-Muñoz R, Gariglio P, Aranda-Anzaldo A. Gene positional changes relative to the nuclear substructure correlate with the proliferating status of hepatocytes during liver regeneration. Nucleic Acids Res 2003; 31:6168 - 79; http://dx.doi.org/10.1093/nar/gkg825; PMID: 14576303
- Elcock LS, Bridger JM. Exploring the effects of a dysfunctional nuclear matrix. Biochem Soc Trans 2008; 36:1378 - 83; http://dx.doi.org/10.1042/BST0361378; PMID: 19021559
- Rivera-Mulia JC, Aranda-Anzaldo A. Determination of the in vivo structural DNA loop organization in the genomic region of the rat albumin locus by means of a topological approach. DNA Res 2010; 17:23 - 35; http://dx.doi.org/10.1093/dnares/dsp027; PMID: 20047947
- Heng HHQ, Goetze S, Ye CJ, Liu G, Stevens JB, Bremer SW, et al. Chromatin loops are selectively anchored using scaffold/matrix-attachment regions. J Cell Sci 2004; 117:999 - 1008; http://dx.doi.org/10.1242/jcs.00976; PMID: 14996931
- Aranda-Anzaldo A. A structural basis for cellular senescence. Aging (Albany NY) 2009; 1:598 - 607; PMID: 20157542
- Alva-Medina J, Dent MAR, Aranda-Anzaldo A. Aged and post-mitotic cells share a very stable higher-order structure in the cell nucleus in vivo. Biogerontology 2010; 11:703 - 16; http://dx.doi.org/10.1007/s10522-010-9285-4; PMID: 20512413
- Smith JR, Hayflick L. Variation in the life-span of clones derived from human diploid cell strains. J Cell Biol 1974; 62:48 - 53; http://dx.doi.org/10.1083/jcb.62.1.48; PMID: 4407044
- Martin GM. Cellular aging--clonal senescence. A review (Part I). Am J Pathol 1977; 89:484 - 512; PMID: 335895
- Jones RB, Whitney RG, Smith JR. Intramitotic variation in proliferative potential: stochastic events in cellular aging. Mech Ageing Dev 1985; 29:143 - 9; http://dx.doi.org/10.1016/0047-6374(85)90014-4; PMID: 3974307
- Takahashi A, Ohtani N, Hara E. Irreversibility of cellular senescence: dual roles of p16INK4a/Rb-pathway in cell cycle control. Cell Div 2007; 2:10; http://dx.doi.org/10.1186/1747-1028-2-10; PMID: 17343761
- Forsyth NR, Elder FFB, Shay JW, Wright WE. Lagomorphs (rabbits, pikas and hares) do not use telomere-directed replicative aging in vitro. Mech Ageing Dev 2005; 126:685 - 91; http://dx.doi.org/10.1016/j.mad.2005.01.003; PMID: 15888323
- Patil CK, Mian IS, Campisi J. The thorny path linking cellular senescence to organismal aging. Mech Ageing Dev 2005; 126:1040 - 5; http://dx.doi.org/10.1016/j.mad.2005.08.001; PMID: 16153470
- Fausto N. Liver regeneration. J Hepatol 2000; 32:Suppl 19 - 31; http://dx.doi.org/10.1016/S0168-8278(00)80412-2; PMID: 10728791
- Michalopoulos GK, DeFrances MC. Liver regeneration. Science 1997; 276:60 - 6; http://dx.doi.org/10.1126/science.276.5309.60; PMID: 9082986
- Schmucker DL, Sanchez H. Liver regeneration and aging: a current perspective. Curr Gerontol Geriatr Res 2011; 2011:526379; http://dx.doi.org/10.1155/2011/526379; PMID: 21912543
- Sigal SM, Gupta S, Gebhard DF Jr., Holst P, Neufeld D. Reid, LM Evidence for a Terminal differentiation process in the rat liver. Differentation 1995; 59:35 - 42; http://dx.doi.org/10.1046/j.1432-0436.1995.5910035.x
- Rehen SK, Yung YC, McCreight MP, Kaushal D, Yang AH, Almeida BS, et al. Constitutional aneuploidy in the normal human brain. J Neurosci 2005; 25:2176 - 80; http://dx.doi.org/10.1523/JNEUROSCI.4560-04.2005; PMID: 15745943
- Yurov YB, Iourov IY, Vorsanova SG, Liehr T, Kolotii AD, Kutsev SI, et al. Aneuploidy and confined chromosomal mosaicism in the developing human brain. PLoS One 2007; 2:e558; http://dx.doi.org/10.1371/journal.pone.0000558; PMID: 17593959
- Mosch B, Morawski M, Mittag A, Lenz D, Tarnok A, Arendt T. Aneuploidy and DNA replication in the normal human brain and Alzheimer’s disease. J Neurosci 2007; 27:6859 - 67; http://dx.doi.org/10.1523/JNEUROSCI.0379-07.2007; PMID: 17596434
- Yang Y, Mufson EJ, Herrup K. Neuronal cell death is preceded by cell cycle events at all stages of Alzheimer’s disease. J Neurosci 2003; 23:2557 - 63; PMID: 12684440
- Gupta S. Hepatic polyploidy and liver growth control. Semin Cancer Biol 2000; 10:161 - 71; http://dx.doi.org/10.1006/scbi.2000.0317; PMID: 10936066
- Gandillet A, Alexandre E, Holl V, Royer C, Bischoff P, Cinqualbre J, et al. Hepatocyte ploidy in normal young rat. Comp Biochem Physiol A Mol Integr Physiol 2003; 134:665 - 73; http://dx.doi.org/10.1016/S1095-6433(02)00374-4; PMID: 12600676
- Sanz N, Díez-Ferńndez C, Alvarez A, Cascales M. Age-dependent modifications in rat hepatocyte antioxidant defense systems. J Hepatol 1997; 27:525 - 34; http://dx.doi.org/10.1016/S0168-8278(97)80358-3; PMID: 9314131
- Solopaev BP, Bobyleva NA. Regeneration of liver with experimental cirrhosis after quadruple resection. Bull Exp Biol Med 1981; 90:1442 - 4; http://dx.doi.org/10.1007/BF00838830
- Sigal SH, Rajvanshi P, Gorla GR, Sokhi RP, Saxena R, Gebhard DR Jr., et al. Partial hepatectomy-induced polyploidy attenuates hepatocyte replication and activates cell aging events. Am J Physiol 1999; 276:G1260 - 72; PMID: 10330018
- Maya-Mendoza A, Herńndez-Muñoz R, Gariglio P, Aranda-Anzaldo A. Natural ageing in the rat liver correlates with progressive stabilisation of DNA-nuclear matrix interactions and withdrawal of genes from the nuclear substructure. Mech Ageing Dev 2005; 126:767 - 82; http://dx.doi.org/10.1016/j.mad.2005.01.011; PMID: 15888332
- Alva-Medina J, Maya-Mendoza A, Dent MAR, Aranda-Anzaldo A. Continued stabilization of the nuclear higher-order structure of post-mitotic neurons in vivo. PLoS One 2011; 6:e21360; http://dx.doi.org/10.1371/journal.pone.0021360; PMID: 21731716
- Thom R. Structural Stability and Morphogenesis: An Outline of a General Theory of Models. Reading, MA: Addison-Wesley,1989; 14.
- Saunders PT. An Introduction to Catastrophe Theory. Cambridge: Cambridge University Press 1995; 17.
- Lambert F. Disorder, a cracked crutch for supporting entropy discussions. J Chem Educ 2002; 79:187 - 92; http://dx.doi.org/10.1021/ed079p187
- Wang N, Tytell JD, Ingber DE. Mechanotransduction at a distance: mechanically coupling the extracellular matrix with the nucleus. Nat Rev Mol Cell Biol 2009; 10:75 - 82; http://dx.doi.org/10.1038/nrm2594; PMID: 19197334
- Aranda-Anzaldo A. On the role of chromatin higher-order structure and mechanical interactions in the regulation of gene expression. Speculations Sci Technol 1989; 12:163 - 76
- Galli C, Guizzardi S, Passeri G, Macaluso GM, Scandroglio R. Life on the wire: on tensegrity and force balance in cells. Acta Biomed 2005; 76:5 - 12; PMID: 16116819
- Ingber DE. Cellular mechanotransduction: putting all the pieces together again. FASEB J 2006; 20:811 - 27; http://dx.doi.org/10.1096/fj.05-5424rev; PMID: 16675838
- Martínez-Ramos I, Maya-Mendoza A, Gariglio P, Aranda-Anzaldo A. A global but stable change in HeLa cell morphology induces reorganization of DNA structural loop domains within the cell nucleus. J Cell Biochem 2005; 96:79 - 88; http://dx.doi.org/10.1002/jcb.20428; PMID: 16052476
- Ludérus ME, van Steensel B, Chong L, Sibon OC, Cremers FF, de Lange T. Structure, subnuclear distribution, and nuclear matrix association of the mammalian telomeric complex. J Cell Biol 1996; 135:867 - 81; http://dx.doi.org/10.1083/jcb.135.4.867; PMID: 8922373
- Marsden MP, Laemmli UK. Metaphase chromosome structure: evidence for a radial loop model. Cell 1979; 17:849 - 58; http://dx.doi.org/10.1016/0092-8674(79)90325-8; PMID: 487432
- Stack SM, Anderson LK. A model for chromosome structure during the mitotic and meiotic cell cycles. Chromosome Res 2001; 9:175 - 98; http://dx.doi.org/10.1023/A:1016690802570; PMID: 11330393
- Sheval EV, Polyakov VY. Visualization of the chromosome scaffold and intermediates of loop domain compaction in extracted mitotic cells. Cell Biol Int 2006; 30:1028 - 40; http://dx.doi.org/10.1016/j.cellbi.2006.07.009; PMID: 17029868
- Anachkova B, Djeliova V, Russev G. Nuclear matrix support of DNA replication. J Cell Biochem 2005; 96:951 - 61; http://dx.doi.org/10.1002/jcb.20610; PMID: 16167334
- Rivera-Mulia JC, Herńndez-Muñoz R, Martínez F, Aranda-Anzaldo A. DNA moves sequentially towards the nuclear matrix during DNA replication in vivo. BMC Cell Biol 2011; 12:3; http://dx.doi.org/10.1186/1471-2121-12-3; PMID: 21244708
- Gilmore EC, Ohshima T, Goffinet AM, Kulkarni AB, Herrup K. Cyclin-dependent kinase 5-deficient mice demonstrate novel developmental arrest in cerebral cortex. J Neurosci 1998; 18:6370 - 7; PMID: 9698328
- Ohshima T, Gilmore EC, Longenecker G, Jacobowitz DM, Brady RO, Herrup K, et al. Migration defects of cdk5(-/-) neurons in the developing cerebellum is cell autonomous. J Neurosci 1999; 19:6017 - 26; PMID: 10407039
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development 1992; 116:201 - 11; PMID: 1483388
- Lemaitre J-M, Danis E, Pasero P, Vassetzky Y, Méchali M. Mitotic remodeling of the replicon and chromosome structure. Cell 2005; 123:787 - 801; http://dx.doi.org/10.1016/j.cell.2005.08.045; PMID: 16325575
- Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma?. Nat Cell Biol 2004; 6:1062 - 7; http://dx.doi.org/10.1038/ncb1104-1062; PMID: 15517000
- Zhang Y, Xi Z, Hegde RS, Shakked Z, Crothers DM. Predicting indirect readout effects in protein-DNA interactions. Proc Natl Acad Sci U S A 2004; 101:8337 - 41; http://dx.doi.org/10.1073/pnas.0402319101; PMID: 15148366
- Gilbert SF. Developmental Biology. Sunderland MA: Sinauer Associates Inc. 2010; 691-692.