Abstract
Natural killer (NK) cells are innate immune effectors that eliminate diseased and tumorigenic targets through the directed secretion of specialized secretory lysosomes, termed lytic granules. This directed secretion is triggered following the formation of an immunological synapse (IS), which is characterized by actin re-modeling and receptor organization at the interface between the NK cell and its susceptible target. Actin at the IS has been described to be permissive to secretion by forming a large central clearance through which lytic granules are released. However we, and others, have recently shown that the actin network in NK cells at the IS is dynamic yet pervasive. These efforts used multiple high resolution imaging techniques to demonstrate that the actin network does not act as a barrier to secretion, but instead enables the secretion of lytic granules through minimally sized clearances. In our recent publication we visualized actin using continuous wave stimulated emission depletion (CW-STED) and lytic granules using the confocal modality. Here we report for the first time dual channel STED nanoscopy of NK cell lytic granules on actin filaments.
As potent effector cells of the innate immune system that rely on germline encoded receptors for activation, NK cells must pass tightly regulated checkpoints to the formation of a mature immunological synapse and subsequent cytotoxicity.Citation1 These checkpoints include the rearrangement of filamentous (F-) actin at the interface between the NK cell and its target, polarization of the MTOC and directed secretion of lytic granules. Characterization of the immunological synapse by 3D reconstruction of confocal images suggested a dense ring of peripheral actin with a paucity of central actin, allowing for secretion of granules through the void in the center.Citation2 However, it has previously been shown that the actin-associated motor protein myosin IIA is required for degranulation in NK cell cytotoxicity.Citation3 In subsequent studies, we determined that myosin IIA is directly associated with NK cell lytic granules and is required for their ability to interact with actin filaments.Citation4 This suggested that granules are associated with actin prior to delivery to the plasma membrane. We hypothesized therefore that F-actin would be present in central regions of the IS and would serve a valuable function in directly interacting with lytic granules. In pursuing this question, we recently demonstrated that F-actin is, indeed present in the central region of the IS, but had been previously undetected due to the limitations of conventional fluorescence microscopy.Citation5,Citation6
While the diffraction barrier of light has previously limited the resolution of microscopy, new advances in imaging have resulted in an explosion of technologies enabling the spatial resolution of structures less than 200 nm.Citation7 One such technology is STED, which employs a toroidal-shaped depletion laser beam that temporarily depletes fluorescent emission around the fluorophore, thus enabling resolution of objects separated by less than 50 nm.Citation8 In our recent work, we employed multiple high-resolution imaging techniques, including total internal reflection fluorescence microscopy, platinum replica electron microscopy and CW-STED to demonstrate comprehensively that F-actin is present throughout the IS.Citation5 In addition, we reported confocal microscopy of lytic granules on actin filaments detected by STED. We have since optimized dual color STED detection and here report the imaging of both NK cell lytic granules and F-actin in STED.
Imaging of Lytic Granules on Actin Filaments in Confocal and Dual Color STED
In order to recapitulate the lytic IS in an alignment suitable for super-resolution imaging, we utilized glass coated with antibodies directed against the NK cell activating receptor NKp30 and adhesion receptor CD18, as described previously.Citation5 The human NK cell line, NK92, was prepared in single cell suspension and adhered to antibody-coated glass for 20 min then fixed. After fixation, cells were permeabilized and stained for F-actin using phalloidin Alexa Fluor 488 and for the lytic granule component perforin using Pacific Orange-conjugated anti-perforin antibody. Using sequential scanning, we evaluated actin via phalloidin Alexa Fluor 488 in STED and anti-perforin via the Pacific Orange secondary antibody in both STED and confocal imaging modes. Images were acquired using Leica ASAF software then exported to Volocity software (Perkin Elmer) and thresholded using the same settings in all cases to allow for quantitative comparison of the images.
As we had previously identified, both F-actin and lytic granules were present throughout the synapse.Citation5 There was a qualitative improvement in the resolution of the lytic granules imaged using the STED modality (, red), when compared with confocal (, red). To quantitatively compare resolution, we measured a single granule in both STED and confocal and determined the full width at half maximum (FWHM) using Leica ASAF software (). FWHM measures the width of the fluorescence intensity peak and thus reflects the ability to separate or resolve objects. As suggested by our observations, analysis confirmed greater resolution in STED, with a FWHM value of 90 nm, whereas FWHM in confocal was 210 nm.
Figure 1. Visualization of lytic granules imaged by CW-STED and confocal on F-actin. NK92 cells were adhered to glass coated with antibody to activating (NKp30) and adhesion (CD18) receptor then fixed, permeabilized and stained for perforin and actin. Cells were imaged using CW-STED (actin, green) and either CW-STED or confocal (perforin, red). Shown is the same cell with granules detected by STED (A) or confocal (B). A region of interest is enlarged to show greater resolution of granules (center panel). (C) Full width half maximum (FWHM) measurements of confocal (green line) and STED images (red line). Horizontal dashed lines show half maxima, vertical dashed lines show width at half maxima. (D) Representative line profile of pixel intensities of actin (green line) and perforin (red line) taken from a line bisecting a single granule (shown in white in STED image enlargement). AU, arbitrary units.
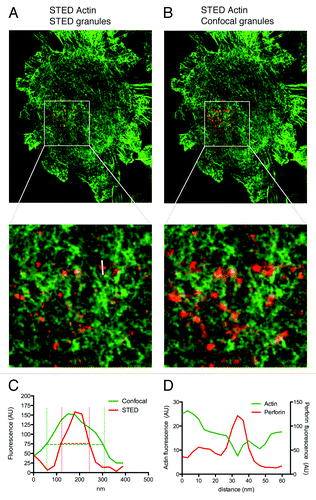
The increased resolution we were able to identify using STED resulted in an ability to define lytic granules of an apparent smaller size. Thus, with this improved ability to distinguish lytic granules, we sought to confirm our earlier finding that lytic granules, while located in areas of F-actin hypodensity, were either in minimally sized clearances in contact with or atop F-actin filaments. In order to accomplish this, we measured line profiles of fluorescence intensity for perforin and F-actin staining. Consistent with our earlier findings, we found an intersection of line profiles (). This indicates that lytic granules are closely associated with F-actin and thus secreted through minimally sized clearances.
Actin reorganization at the IS is a critical prerequisite for cytotoxicity in both adaptive and innate immune effector cells.Citation9-Citation12 It is required for cell surface receptor rearrangements, cell activation signaling and for the subsequent polarization of lytic granules to the IS. Previous studies performed using 3D reconstruction of confocal images, however, have resulted in a model for secretion in which lytic granules are expelled through a central clearance of actin in both NK cells and their adaptive counterpart, the cytotoxic T lymphocyte.Citation2,Citation3,Citation13,Citation14 Detailed super-resolution analysis of the NK cell lytic synapse by two independent laboratories suggests a new paradigm for cytotoxicity in which a pervasive actin network is present and acts not as a barrier but a facilitator for secretion.Citation5,Citation6 With this new understanding it will be interesting to see if this model extends to other immune cells undergoing directed secretion of both specialized secretory lysosomes and cytokines. Alternatively, it may represent an additional checkpoint utilized by cells of the innate immune system as they access pre-armed functions. Using dual color STED nanoscopy of lytic granules on actin filaments in NK cells we have shown in unprecedented resolution details of this new paradigm.
Acknowledgments
This work was supported by NIH R01 AI67946 (awarded to J.S.O.). The authors wish to thank Geoff Daniels at Leica Microsystems for technical assistance and Dr. G. Rak for helpful discussion.
References
- Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol 2008; 8:713 - 25; http://dx.doi.org/10.1038/nri2381; PMID: 19172692
- Orange JS, Harris KE, Andzelm MM, Valter MM, Geha RS, Strominger JL. The mature activating natural killer cell immunologic synapse is formed in distinct stages. Proc Natl Acad Sci U S A 2003; 100:14151 - 6; http://dx.doi.org/10.1073/pnas.1835830100; PMID: 14612578
- Andzelm MM, Chen X, Krzewski K, Orange JS, Strominger JL. Myosin IIA is required for cytolytic granule exocytosis in human NK cells. J Exp Med 2007; 204:2285 - 91; http://dx.doi.org/10.1084/jem.20071143; PMID: 17875677
- Sanborn KB, Rak GD, Maru SY, Demers K, Difeo A, Martignetti JA, et al. Myosin IIA associates with NK cell lytic granules to enable their interaction with F-actin and function at the immunological synapse. J Immunol 2009; 182:6969 - 84; http://dx.doi.org/10.4049/jimmunol.0804337; PMID: 19454694
- Rak GD, Mace EM, Banerjee PP, Svitkina T, Orange JS. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol 2011; 9:e1001151; http://dx.doi.org/10.1371/journal.pbio.1001151; PMID: 21931536
- Brown AC, Oddos S, Dobbie IM, Alakoskela JM, Parton RM, Eissmann P, et al. Remodelling of cortical actin where lytic granules dock at natural killer cell immune synapses revealed by super-resolution microscopy. PLoS Biol 2011; 9:e1001152; http://dx.doi.org/10.1371/journal.pbio.1001152; PMID: 21931537
- Schermelleh L, Heintzmann R, Leonhardt H. A guide to super-resolution fluorescence microscopy. J Cell Biol 2010; 190:165 - 75; http://dx.doi.org/10.1083/jcb.201002018; PMID: 20643879
- Hell SW. Far-field optical nanoscopy. Science 2007; 316:1153 - 8; http://dx.doi.org/10.1126/science.1137395; PMID: 17525330
- Orange JS, Ramesh N, Remold-O’Donnell E, Sasahara Y, Koopman L, Byrne M, et al. Wiskott-Aldrich syndrome protein is required for NK cell cytotoxicity and colocalizes with actin to NK cell-activating immunologic synapses. Proc Natl Acad Sci U S A 2002; 99:11351 - 6; http://dx.doi.org/10.1073/pnas.162376099; PMID: 12177428
- Wulfing C, Purtic B, Klem J, Schatzle JD. Stepwise cytoskeletal polarization as a series of checkpoints in innate but not adaptive cytolytic killing. Proc Natl Acad Sci U S A 2003; 100:7767 - 72; http://dx.doi.org/10.1073/pnas.1336920100; PMID: 12802007
- Valitutti S, Dessing M, Aktories K, Gallati H, Lanzavecchia A. Sustained signaling leading to T cell activation results from prolonged T cell receptor occupancy. Role of T cell actin cytoskeleton. J Exp Med 1995; 181:577 - 84; http://dx.doi.org/10.1084/jem.181.2.577; PMID: 7836913
- Wülfing C, Sjaastad MD, Davis MM. Visualizing the dynamics of T cell activation: intracellular adhesion molecule 1 migrates rapidly to the T cell/B cell interface and acts to sustain calcium levels. Proc Natl Acad Sci U S A 1998; 95:6302 - 7; http://dx.doi.org/10.1073/pnas.95.11.6302; PMID: 9600960
- Stinchcombe JC, Bossi G, Booth S, Griffiths GM. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 2001; 15:751 - 61; http://dx.doi.org/10.1016/S1074-7613(01)00234-5; PMID: 11728337
- Stinchcombe JC, Majorovits E, Bossi G, Fuller S, Griffiths GM. Centrosome polarization delivers secretory granules to the immunological synapse. Nature 2006; 443:462 - 5; http://dx.doi.org/10.1038/nature05071; PMID: 17006514