Abstract
Candida dubliniensis, an emerging fungal pathogen, is the closest known species to the established pathogenic species Candida albicans. Despite the fact that these two species share > 80% genome sequence identity, they exhibit distinct properties such as less hyphal growth, reduced pathogenicity and increased sensitivity to sodium stress and elevated temperatures in C. dubliniensis compared with C. albicans. It is, however, largely unknown whether signaling pathways are conserved in the two Candida species. Calcineurin signaling is known to be required for hyphal growth in Cryptococcus neoformans and Aspergillus fumigatus but remains elusive in C. albicans. Our recent study showed that calcineurin plays a clearly demonstrable role in controlling hyphal growth, drug tolerance and virulence in C. dubliniensis. Here, we extend our studies and show that calcineurin is conserved in controlling endoplasmic reticulum stress but distinct in governing pH homeostasis. Furthermore, we demonstrate that azole or echinocandin drugs in combination with the calcineurin inhibitor FK506 exhibit a synergistic effect against C. dubliniensis wild-type and echinocandin-resistant strains. The involvement of calcineurin in a variety of fungal virulence attributes and as a target for fungicidal synergism with azoles and echinocandins highlights the potential of combination therapy with calcineurin inhibitors for treating Candida infections.
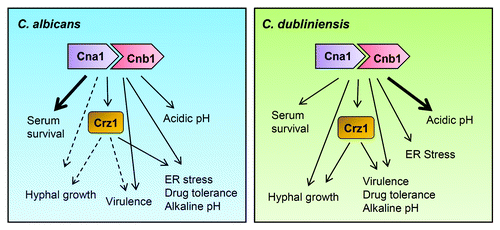
Calcineurin is a eukaryotic Ca2+-calmodulin-dependent serine/threonine specific protein phosphatase that is required for fungal virulence in a variety of human fungal pathogens including C. albicans,Citation1-Citation3 C. neoformans,Citation4,Citation5 and A. fumigatus.Citation6 We recently investigated the role of calcineurin in Candida dubliniensis, a sibling species of C. albicans that often infects the oral cavities of immunocompromised patients, such as those with HIV/AIDS.Citation7,Citation8 In C. dubliniensis, we found that calcineurin is required for cell wall integrity, hyphal growth, serum survival and virulence.Citation9 Calcineurin played a greater role in serum survival of C. albicans compared with C. dubliniensisCitation9 (). Similar to results in C. albicans, the calcineurin downstream target Crz1 was not required for serum survival in C. dubliniensis. Furthermore, calcineurin and crz1/crz1 mutants had reduced tolerance to azole and echinocandin antifungal drugs in both species ().
Figure 1. Comparison of the calcineurin pathway in C. albicans and C. dubliniensis. The roles of calcineurin and Crz1 in hyphal growth are unclear in C. albicans (dotted arrows) while their roles in hyphal growth of C. dubliniensis (right panel) are demonstrable. Calcineurin is required for virulence in both Candida species. ER stress responses are controlled by Crz1-dependent and Crz1-independent calcineurin signaling in C. albicans and C. dubliniensis, respectively. Drug tolerance and alkaline pH homeostasis are governed by the Crz1-dependent calcineurin pathway in both Candida species. C. dubliniensis calcineurin plays a larger role (bold arrow) in controlling acidic pH homeostasis compared with C. albicans. In C. albicans calcineurin plays a greater role (bold arrow) in serum survival compared with C. dubliniensis.
While C. albicans is still the major cause of Candida infections, C. dubliniensis now causes 2 to 7% of all clinical cases of candidemia.Citation10,Citation11 C. dubliniensis isolates are susceptible to azole antifungal drugs but can rapidly develop resistance during clinical therapy. Thus, it is important to explore novel therapies and drug combinations for treatment. Studies in C. albicans have shown that calcineurin is required for azole and/or echinocandin tolerance.Citation12 Here, we demonstrate that FK506 exhibits synergism with fluconazole, posaconazole, and caspofungin based on in vitro minimum inhibitory concentration (MIC) and checkerboard assays against a wild-type and an echinocandin-resistant strain, suggesting the potential for drug combination therapy.
We are also interested in further characterizing the roles of calcineurin, especially with respect to endoplasmic reticulum (ER) stress and pH homeostasis. The ability to switch to hyphal growth in response to ER stress and to maintain pH homeostasis are both critical for virulence of C. albicans.Citation13 Previously, we found that calcineurin and Crz1 are required for hyphal growth in C. dubliniensis on nutrient limiting media.Citation9 However, the roles of calcineurin and Crz1 in C. albicans hyphal growth remain unclear with conflicting data reported on possible roles of calcineurin and Crz1 in C. albicans morphogenesis ().Citation2,Citation3,Citation12,Citation14 Here, we characterize two stress responses associated with hyphal growth in C. dubliniensis and C. albicans.
Calcineurin is Essential for ER Stress Response in C. dubliniensis and C. albicans
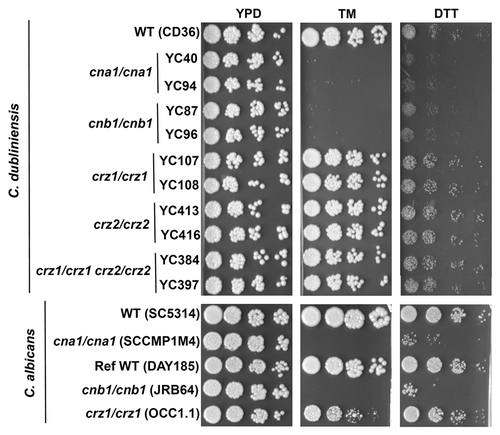
ER stress due to an accumulation of unfolded or misfolded proteins activates the unfolded protein response (UPR) signaling pathway in eukaryotic cellsCitation15,Citation16 and has been shown to be involved in C. albicans morphogenesis.Citation17 UPR signaling activates a variety of downstream transcripts, including the bZIP transcription factor Hac1 that impacts C. albicans hyphal growth in response to ER stress.Citation17 Tunicamycin (TM) and dithiothreitol (DTT) induce the UPR by inhibiting N-linked glycosylation of secreted proteins and preventing disulfide bond formation, respectively.Citation16 We found that C. albicans and C. dubliniensis calcineurin mutants are hypersensitive to tunicamycin and DTT compared with the wild-type (). Furthermore, the role of Crz1 in ER stress is different in C. albicans compared with C. dubliniensis (). While C. dubliniensis crz1/crz1, crz2/crz2, and crz1/crz1 crz2/crz2 double mutants grew similarly to wild-type in the presence of TM, the C. albicans crz1/crz1 mutant exhibited attenuated growth that was intermediate between wild-type and calcineurin mutants ( and ), indicating a divergent function of Crz1 in ER stress responses in both Candida species. Interestingly, we found that the C. dubliniensis wild-type strainis hypersensitive to the ER stress inducer DTT compared with the C. albicans wild-type strain (), indicating that the tolerance to protein misfolding has been reduced in C. dubliniensis compared with C. albicans.
Figure 2. Calcineurin functions are conserved whereas Crz1 has different roles during ER stress in C. albicans and C. dubliniensis. C. dubliniensis and C. albicans calcineurin mutants were hypersensitive to the ER stress inducers tunicamycin (TM) and dithiothreitol (DTT). The C. albicans crz1/crz1 mutants, but not C. dubliniensis crz1/crz1 mutants, exhibit TM sensitivity intermediate between wild-type and calcineurin mutants. Cells were grown overnight in YPD medium at 24°C, washed twice in dH2O, 5-fold serially diluted, and spotted onto YPD medium containing TM (1 µg/ml) or DTT (45 mM). All strains were spotted on the same media. Strains tested are described in reference.Citation9
Calcineurin Plays a Greater Role in Acidic pH Homeostasis in C. dubliniensis Compared with C. albicans
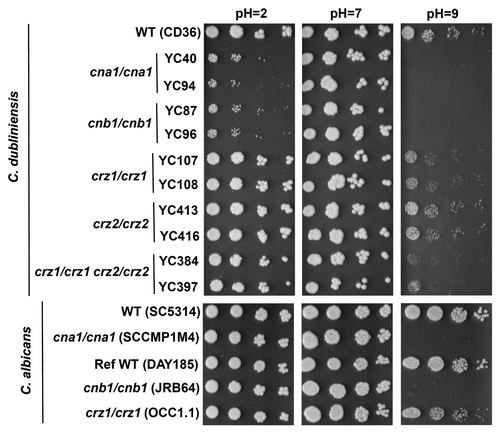
Another crucial factor for hyphal growth and virulence of C. albicans is the ability to respond to environmental pH changes. Both calcineurin and Crz1 have been demonstrated to play a role in C. albicans tolerance to changes in environmental pH through the Rim101/pacC pH-sensing pathway.Citation18,Citation19 Previoius studies have shown that Rim101 acts in parallel with calcineurin and Crz1 to allow for adaption to alkaline pH in C. albicans.Citation19 Furthermore, Crz1, Crz2, and calcineurin are needed for adaptation to acidic pH in C. albicans. The role of calcineurin in pH homeostasis of C. dubliniensis was unknown. C. dubliniensis forms true hyphae less efficiently than C. albicans in response to pH shifts in vitro.Citation20,Citation21 Here, we show that C. dubliniensis cna1/cna1 and cnb1/cnb1 mutants are hypersensitive to acidic and alkaline pHs compared with wild-type (), whereas C. albicans calcineurin mutants did not show attenuated growth at acidic pH. This result differs from previous studies where calcineurin was shown to be required for growth at acidic pHCitation19 and may be attributable to the use of different pH buffering systems or experimental procedures. Taken together, we conclude that in C. dubliniensis calcineurin plays a greater role in acidic pH homeostasis compared with its role in C. albicans ( and ). Interestingly, while C. dubliniensis and C. albicans crz1/crz1 mutants grew similarly to wild-type at acidic pH, they exhibited attenuated growth that was intermediate between wild-type and calcineurin mutants at alkaline pH. However, C. dubliniensis crz2/crz2 mutants did not have growth defects in acidic or alkaline pH (). These results suggest that alkaline pH homeostasis is controlled by the Crz1-dependent calcineurin pathway in C. dubliniensis and C. albicans, whereas acidic pH homeostasis is governed by a Crz1-independent calcineurin pathway in C. dubliniensis.
Figure 3. Calcineurin plays a greater role in controlling acidic pH response in C. dubliniensis. Calcineurin is essential for growth at alkaline conditions (pH = 9) in C. dubliniensis and C. albicans. While C. dubliniensis calcineurin mutants had attenuated growth at acidic pH, the C. albicans calcineurin mutants did not. Cells were grown overnight in YPD medium at 24°C, washed twice in dH2O, 5-fold serially diluted, and spotted onto YPD medium containing 150 mM HEPES buffered at pH 2 or 7. For pH 9 medium, YPD was buffered with 150 mM pH 14 HEPES (85 ml of YPD plus 15 ml of 1M HEPES at pH 14). The pHs of solid media were confirmed with pH indicator strips (Sigma-Aldrich, Z134147). All strains were spotted on the same media.
Calcineurin Inhibitor Exhibits Synergism with Caspofungin against C. dubliniensis Echinocandin-resistant Strain
To determine if the calcineurin inhibitor FK506 exhibits synergism with caspofungin against the echinocandin-resistant strain DPL278,Citation22 we performed disk diffusion and checkerboard assays following the procedures described by the Clinical and Laboratory Standards Institute (CLSI) protocol M27-A3. The DPL278 strain is resistant to caspofungin but susceptible to azoles ( and ). We further found that the resistance to caspofungin of the DPL278 strain was reversed by supplementation with FK506 ( and ). Meanwhile, we demonstrated that FK506 exhibits synergism with caspofungin, fluconazole, or posaconazole against C. dubliniensis wild-type and echinocandin-resistant strain [fractional inhibitory concentrations (FIC) < 0.5, ], suggesting that these drug combinations have therapeutic potential for C. dubliniensis infections.
Figure 4. Calcineurin inhibitor exhibits synergism with caspofungin against wild-type and echinocandin-resistant C. dubliniensis strain. Disk diffusion assays were used to determine echinocandin and azole susceptibility of a wild-type (CD36) and an echinocandin-resistant strain (DPL278). Cells were grown overnight at 30°C, and cells at a density of 0.1 OD600 (in 100 µl) were spread on the surface of YPD medium in the absence or presence of FK506 (1 µg/ml). The disk (6 mm) containing 5 µl of caspofungin (CAS, 5 µg), posaconazole (PSC, 1 µg), or fluconazole (FLC, 10 µg) was placed on the surface of the plates, and incubated at 30°C for 48 h.
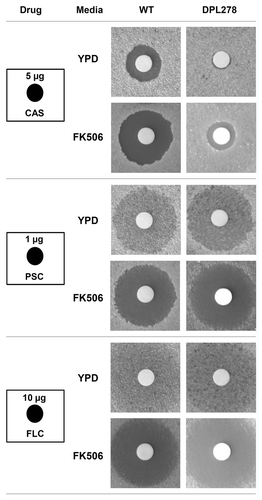
Table 1. FK506 exhibits synergism with azole or echinocandin drugs against C. dubliniensis wild-type and echinocandin-resistant strains
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Cecelia Shertz and Joanne Kingsbury for edits and comments on the manuscript, and David Perlin and Ana Alastruey-Izquierdo at the Public Health Research Institute Center of UMDNJ-New Jersey Medical School for the C. dubliniensis echinocandin- resistant strain DPL278. These studies were funded by a Duke University Chemistry Department Undergraduate Research Fellowship (J.Z.), the Center for AIDS Research (CFAR grant, 2P30 AI064518–06 to Y.-L.C.), NIH/NIAID R01 grant AI50438 (J.H.), and pilot funds from Merck and Co. Inc. and Astellas Pharma Inc. (J.H. and Y.-L.C.).
References
- Blankenship JR, Heitman J. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect Immun 2005; 73:5767 - 74; http://dx.doi.org/10.1128/IAI.73.9.5767-5774.2005; PMID: 16113294
- Blankenship JR, Wormley FL, Boyce MK, Schell WA, Filler SG, Perfect JR, et al. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot Cell 2003; 2:422 - 30; http://dx.doi.org/10.1128/EC.2.3.422-430.2003; PMID: 12796287
- Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol Microbiol 2003; 48:959 - 76; http://dx.doi.org/10.1046/j.1365-2958.2003.03495.x; PMID: 12753189
- Fox DS, Cruz MC, Sia RAL, Ke H, Cox GM, Cardenas ME, et al. Calcineurin regulatory subunit is essential for virulence and mediates interactions with FKBP12-FK506 in Cryptococcus neoformans. Mol Microbiol 2001; 39:835 - 49; http://dx.doi.org/10.1046/j.1365-2958.2001.02295.x; PMID: 11251806
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J 1997; 16:2576 - 89; http://dx.doi.org/10.1093/emboj/16.10.2576; PMID: 9184205
- Steinbach WJ, Cramer RA Jr., Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, et al. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell 2006; 5:1091 - 103; http://dx.doi.org/10.1128/EC.00139-06; PMID: 16835453
- Moran GP, Sullivan DJ, Henman MC, McCreary CE, Harrington BJ, Shanley DB, et al. Antifungal drug susceptibilities of oral Candida dubliniensis isolates from human immunodeficiency virus (HIV)-infected and non-HIV-infected subjects and generation of stable fluconazole-resistant derivatives in vitro. Antimicrob Agents Chemother 1997; 41:617 - 23; PMID: 9056003
- Perea S, López-Ribot JL, Wickes BL, Kirkpatrick WR, Dib OP, Bachmann SP, et al. Molecular mechanisms of fluconazole resistance in Candida dubliniensis isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother 2002; 46:1695 - 703; http://dx.doi.org/10.1128/AAC.46.6.1695-1703.2002; PMID: 12019078
- Chen YL, Brand A, Morrison EL, Silao FG, Bigol UG, Malbas FF Jr., et al. Calcineurin controls drug tolerance, hyphal growth, and virulence in Candida dubliniensis. Eukaryot Cell 2011; 10:803 - 19; http://dx.doi.org/10.1128/EC.00310-10; PMID: 21531874
- Jabra-Rizk MA, Johnson JK, Forrest G, Mankes K, Meiller TF, Venezia RA. Prevalence of Candida dubliniensis fungemia at a large teaching hospital. Clin Infect Dis 2005; 41:1064 - 7; http://dx.doi.org/10.1086/432943; PMID: 16142677
- Sullivan DJ, Moran GP, Pinjon E, Al-Mosaid A, Stokes C, Vaughan C, et al. Comparison of the epidemiology, drug resistance mechanisms, and virulence of Candida dubliniensis and Candida albicans. FEMS Yeast Res 2004; 4:369 - 76; http://dx.doi.org/10.1016/S1567-1356(03)00240-X; PMID: 14734017
- Chen YL, Kozubowski L, Cardenas ME, Heitman J. On the roles of calcineurin in fungal growth and pathogenesis. Curr Fungal Infect Rep 2010; 4:244 - 55; http://dx.doi.org/10.1007/s12281-010-0027-5
- Calderone RA, Fonzi WA. Virulence factors of Candida albicans. Trends Microbiol 2001; 9:327 - 35; http://dx.doi.org/10.1016/S0966-842X(01)02094-7; PMID: 11435107
- Bader T, Schröppel K, Bentink S, Agabian N, Köhler G, Morschhäuser J. Role of calcineurin in stress resistance, morphogenesis, and virulence of a Candida albicans wild-type strain. Infect Immun 2006; 74:4366 - 9; http://dx.doi.org/10.1128/IAI.00142-06; PMID: 16790813
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 2007; 8:519 - 29; http://dx.doi.org/10.1038/nrm2199; PMID: 17565364
- Travers KJ, Patil CK, Wodicka L, Lockhart DJ, Weissman JS, Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell 2000; 101:249 - 58; http://dx.doi.org/10.1016/S0092-8674(00)80835-1; PMID: 10847680
- Wimalasena TT, Enjalbert B, Guillemette T, Plumridge A, Budge S, Yin Z, et al. Impact of the unfolded protein response upon genome-wide expression patterns, and the role of Hac1 in the polarized growth, of Candida albicans. Fungal Genet Biol 2008; 45:1235 - 47; http://dx.doi.org/10.1016/j.fgb.2008.06.001; PMID: 18602013
- Davis D, Edwards JE Jr., Mitchell AP, Ibrahim AS. Candida albicans RIM101 pH response pathway is required for host-pathogen interactions. Infect Immun 2000; 68:5953 - 9; http://dx.doi.org/10.1128/IAI.68.10.5953-5959.2000; PMID: 10992507
- Kullas AL, Martin SJ, Davis D. Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Mol Microbiol 2007; 66:858 - 71; http://dx.doi.org/10.1111/j.1365-2958.2007.05929.x; PMID: 17927701
- Stokes C, Moran GP, Spiering MJ, Cole GT, Coleman DC, Sullivan DJ. Lower filamentation rates of Candida dubliniensis contribute to its lower virulence in comparison with Candida albicans. Fungal Genet Biol 2007; 44:920 - 31; http://dx.doi.org/10.1016/j.fgb.2006.11.014; PMID: 17251042
- Moran GP, MacCallum DM, Spiering MJ, Coleman DC, Sullivan DJ. Differential regulation of the transcriptional repressor NRG1 accounts for altered host-cell interactions in Candida albicans and Candida dubliniensis. Mol Microbiol 2007; 66:915 - 29; http://dx.doi.org/10.1111/j.1365-2958.2007.05965.x; PMID: 17927699
- Arendrup MC, Park S, Brown S, Pfaller M, Perlin DS. Evaluation of CLSI M44-A2 disk diffusion and associated breakpoint testing of caspofungin and micafungin using a well-characterized panel of wild-type and fks hot spot mutant Candida isolates. Antimicrob Agents Chemother 2011; 55:1891 - 5; http://dx.doi.org/10.1128/AAC.01373-10; PMID: 21357293