Abstract
One of the most successful invasive species is the common wasp, Vespula vulgaris. We recently reported how foragers of this species have adopted previously unknown interference behavior when competing for food with native ants. Picking their opponents up in their mandibles, flying backward and dropping them some distance away from the disputed resource, wasps were shown to efficiently deal with a yet aggressive competitor and to modulate this behavior according to circumstances. Here we further discuss the nature and functioning of this unusual strategy. We first highlight the questions this interaction raises regarding the competitive advantages offered by asymmetries in body size and flight ability. Then, we argue that this study system illustrates the important role of behavioral plasticity in biological invasions; not only in the success of invaders but also in the ability of native species to coexist with these invaders.
Invasive insect species can affect native ecosystems through a variety of mechanisms. Many of these mechanisms are still not clearly understood. In particular, generalist predators may exert especially widespread and pervasive effects on native communities due to the complexity of their trophic position.Citation1,Citation2 This is the case of the common wasp Vespula vulgaris (Vespidae), which was accidentally introduced in New Zealand and is now one the most ecologically problematic biological invaders in the country.Citation3 In a recent study, we showed that these wasps can directly affect the foraging ability of native species. When competing for protein resources with native ants Prolasius advenus (Formicinae), the wasps pick the ants up using their mandibles, fly backward and drop them some distance away from the food items.Citation4 In the present article, this previously unknown interference behavior is discussed in the light of two main concepts: asymmetric competition and behavioral plasticity.
Interference competition has already been shown between ants and larger animals other than wasps. For example, aggressive ants can exclude hermit crabs from carrion, geckos from fruits, or birds from trees.Citation5-Citation8 In these examples, no attempt of ant removal was reported and avoidance appears to be the main response by the crabs, geckos and birds. The general view that in interference competition larger animals are superior to smaller ones is thus contradicted by these examples, while it is in line with our study showing ant-removal by wasps.Citation4,Citation8,Citation9 Many different factors undoubtedly constrain or influence the decisions of taxonomically distant organisms competing with ants. One of them, however, may be recurrent: it relies on the fact that many ant species, including P. advenus and those examined in the above-cited studies, use chemical substances in aggressive interactions. As a result, killing or physically removing ant workers from a shared resource presents a high risk of contact with formic acid and other harmful or repellent substances. We observed that wasps appeared to try to lower this risk by usually seizing the ants from the dorsal side of their thorax and by avoiding crushing them, although their mandible size and strength would easily permit a wasp to crush or completely severe an ants abdomen from its thorax.Citation4 Such a finely adjusted ant-removal strategy may be impossible in larger animals like small vertebrates. Thus, ant-removal as an interference strategy may be restricted to animals that are large enough to allow for a size advantage, but small enough so that the ant defenses can be identified and avoided. In other words, the competitive advantages may not be necessarily linearly proportional to the degree of size asymmetry for solitary foragers interfering with ants.
Social wasps may therefore present a degree of size asymmetry relative to ants that, along with their flight ability, could favor the development of ant-dropping as a common competitive strategy in these insects. Although it is currently known only from V. vulgaris and P. advenus so far, there are several reasons to assume that other species may display the same strategy. For example, several species of Polistine wasps use a similar behavior as a form of nest defense again predatory attacks by ants, dropping scout workers away from their nests.Citation10 Some bees also seem to be able to use a similar defensive tactic.Citation11 More generally, picking up and carrying a competitor away from the disputed resource appears to be such an efficient interference strategy that it is tempting to speculate that similar behaviors may have emerged in other ecological systems involving at least one flying competitor. In support of this assumption is an anecdotal observation of two bird species differing in size and competing for food.Citation12 Here, a bird individual from the bigger species was repeatedly observed to “pick up [its smaller opponent] and drop it off the side of the feeder” to which both species were attracted, so quickly that the smaller bird “had no chance to struggle before it was dropped.” Whether in different wasp species or in other distant taxa, we think that the search of similar interference behaviors is to be encouraged, as they could provide a model for studying the evolution of homologous and/or analogous competitive behaviors related to flight ability and size asymmetry.
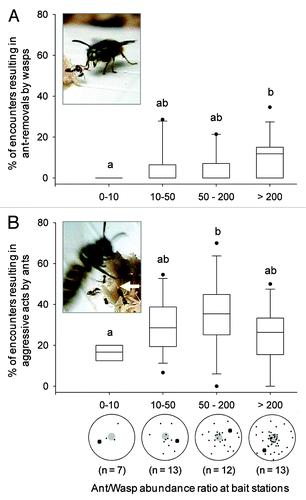
This ant-dropping behavior also illustrates how interactions between native and invasive species may be mediated by plasticity. In social wasps, the invasive success and ecological impacts of V. pensylvanica in Hawaii have been linked to plasticity of life history traits.Citation13 Our study suggests that flexibility in competitive behavior is another type of plasticity that may promote the success of invasive Vespula wasps. Increased ant abundance seemed to cause fewer wasps to visit food resources, but wasps that did compete with increasing densities of ants increased their competitive efforts so they can get at least a brief access to the resource. Indeed, the more ant competitors at food baits, the more frequently ant-removals occurred () and the further away ants were dropped.Citation4 These results suggest that wasps are able to assess the degree of competition and modify their behavior accordingly. Interestingly, ants themselves also seem to adjust their competitive behavior to the circumstances. Further examination of our results revealed that the proportion of interactions in which ants behaved aggressively toward wasps varied as a function of the ratio between average ant and wasp abundances. The frequency of aggressive acts by ants was the highest with an ant/wasp ratio of between 50 and 200, but tended to be lower both below and above these values (). An explanation for such a pattern could be that aggressiveness in P. advenus workers is dependent on group size and competitive pressure, as it is in other Formicine species.Citation14,Citation15 As a result, ants would be less aggressive toward wasps when perceiving either (1) that they are not numerous enough to efficiently face the threat posed by many individual wasps, and/or (2) that on the contrary they have secured the food item through massive recruitment, making wasps visits increasingly rare and costly aggressive behavior redundant. Additional work is currently being conducted by our team to examine in detail how the intensity of ant aggression, and also the exact amount of food collected by ants and wasps, varies according to the abundance of both species. The results obtained so far, however, suggest that behavioral plasticity characterizes responses of both invasive wasps and these native ants. This plasticity can be hypothesized to promote their coexistence, in line with more general views about the ecological consequences of phenotypic plasticity.Citation16 A reduced level of competitive or aggressive responses when the context makes such behaviors useless or inefficient may result in both ants and wasps taking the advantage alternatively, enabling some degree of food collection for both. Accordingly to this hypothesis, the two species were usually not seen to completely exclude each other from food resources, and abundant populations of native ants and invasive wasps can co-exist (unpublished data).
Figure 1. Percentage of ant-wasp encounters resulting in aggressive acts by wasps (A) or ants (B), as a function of the ratio between average ant and wasp abundance at protein baits (n = number of bait stations per ratio category). Box plots show 10th and 90th percentiles (whiskers), 25th and 75th percentiles (boundary of the box), median (line) and outliers (black dots). All data were obtained by videotaping ant-wasp interactions at each bait station for approximately 40 min (see ref. Citation4 for details). Inset pictures show the typical postures of (A) wasps just before picking up an ant and dropping it away from the resource, or (B) ants adopting a threatening posture with wide open mandibles and a drop of acid at the tip of the gaster (white arrow). Below the x-axis of (B) is a schematic representation of ants and wasps (small and large black dots, respectively) when both species were present around the food bait (large gray dot). The proportion of aggressive interactions is relative to the number of passive contacts (contacts that resulted in no response from either species). This proportion differed significantly in both wasps and ants according to the category of ant/wasp abundance ratio (Kruskal-Wallis tests: H = 9.42, p = 0.024 and H = 8.43, p = 0.038; respectively). Different letters indicate a significant difference after a Dunn’s post-hoc test (p < 0.05).
Several authors have pointed out that an increased consideration of behavior in general, and behavioral plasticity in particular, would enhance our ability to assess and predict the ecological success of introduced species.Citation17-Citation20 The present work supports these ideas, and further suggests that such considerations may be similarly crucial to understand the level of biotic resistance displayed by native species confronted to biological invaders.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
This work was supported by a RSNZ Marsden Fund grant.
References
- Kenis M, Auger-Rozenberg MA, Roques A, Timms L, Péré C, Cock MJW, et al. Ecological effects of invasive alien insects. Biol Inv 2009; 11:21 - 45; http://dx.doi.org/10.1007/s10530-008-9318-y
- Crowder DW, Snyder WE. Eating their way to the top? Mechanisms underlying the success of invasive insect generalist predators. Biol Inv 2010; 12:2857 - 76; http://dx.doi.org/10.1007/s10530-010-9733-8
- Beggs JR. The ecological consequences of social wasps (Vespula spp.) invading an ecosystem that has an abundant carbohydrate resource. Biol Conserv 2001; 99:17 - 28; http://dx.doi.org/10.1016/S0006-3207(00)00185-3
- Grangier J, Lester PJ. A novel interference behaviour: invasive wasps remove ants from resources and drop them from a height. Biol Lett 2011; 7:664 - 7; http://dx.doi.org/10.1098/rsbl.2011.0165; PMID: 21450726
- McNatty A, Abbott KL, Lester PJ. Invasive ants compete with and modify the trophic ecology of hermit crabs on tropical islands. Oecologia 2009; 160:187 - 94; http://dx.doi.org/10.1007/s00442-009-1279-z; PMID: 19214589
- Hansen DM, Müller CB. Invasive ants disrupt gecko pollination and seed dispersal of the endangered plant Roussea simplex in Mauritius. Biotropica 2009; 41:202 - 8; http://dx.doi.org/10.1111/j.1744-7429.2008.00473.x
- Haemig PD. Interference from ants alters foraging ecology of great tits. Behav Ecol Sociobiol 1996; 38:25 - 9; http://dx.doi.org/10.1007/s002650050213
- Aho T, Kuitunen M, Suhonen J, Jäntti A, Hakkari T. Behavioural responses of Eurasian treecreepers, Certhia familiaris, to competition with ants. Anim Behav 1997; 54:1283 - 90; http://dx.doi.org/10.1006/anbe.1997.0547; PMID: 9398381
- Persson L. Asymmetrical competition: are larger animals competitively superior?. Am Nat 1985; 126:261 - 6; http://dx.doi.org/10.1086/284413
- London KB, Jeanne RL. The interaction between mode of colony founding, nest architecture and ant defense in polistine wasps. Ethol Ecol Evol 2000; 12:13 - 25
- Duangphakdee O, Koeniger N, Koeniger G, Wongsiri S, Deowanish S. Reinforcing a barrier – a specific social defense of the dwarf honeybee (Apis florae) released by the weaver ant (Oecophylla smaragdina). Apidologie (Celle) 2005; 36:505 - 11; http://dx.doi.org/10.1051/apido:2005036
- Paulson DR. Unusual agonistic behavior in a Green Honeycreeper. Wilson Bull 1988; 100:503 - 4
- Wilson EE, Mullen LM, Holway DA. Life history plasticity magnifies the ecological effects of a social wasp invasion. Proc Natl Acad Sci U S A 2009; 106:12809 - 13; http://dx.doi.org/10.1073/pnas.0902979106; PMID: 19625616
- Tanner CJ. Numerical assessment affects aggression and competitive ability: a team-fighting strategy for the ant Formica xerophila. Proc Biol Sci 2006; 273:2737 - 42; http://dx.doi.org/10.1098/rspb.2006.3626; PMID: 17015327
- Tanner CJ. Resource characteristics and competition affect colony and individual foraging strategies of the wood ant Formica integroides. Ecol Entomol 2008; 33:127 - 36; http://dx.doi.org/10.1111/j.1365-2311.2007.00950.x
- Miner BG, Sultan SE, Morgan SG, Padilla DK, Relyea RA. Ecological consequences of phenotypic plasticity. Trends Ecol Evol 2005; 20:685 - 92; http://dx.doi.org/10.1016/j.tree.2005.08.002; PMID: 16701458
- Holway DA, Suarez AV. Animal behavior: an essential component of invasion biology. Trends Ecol Evol 1999; 14:328 - 30; http://dx.doi.org/10.1016/S0169-5347(99)01636-5; PMID: 10407433
- Sagata K, Lester PJ. Behavioural plasticity associated with propagule size, resources, and the invasion success of the Argentine ant Linepithema humile. J Appl Ecol 2009; 46:19 - 27; http://dx.doi.org/10.1111/j.1365-2664.2008.01523.x
- Wright TF, Eberhard JR, Hobson EA, Avery ML, Russello MA. Behavioral flexibility and species invasions: the adaptive flexibility hypothesis. Ethol Ecol Evol 2010; 22:393 - 404; http://dx.doi.org/10.1080/03949370.2010.505580
- Chapple DG, Simmonds SM, Wong BBM. Can behavioural and personality traits influence the success of unintentional species introduction?. Trends Ecol Evol 2012; 27:57 - 64; http://dx.doi.org/10.1016/j.tree.2011.09.010; PMID: 22001529