Abstract
Neuronal polarization, the process by which neurons form multiple dendrites and an axon from the soma, is the first critical step in the formation and function of neural networks. Polarization begins with the rapid extension of a single neurite to produce an axon of impressive size and complex geometry, while the remaining sister neurites differentiate into dendrites. The extensive biosynthesis required to produce an axon therefore necessitates coordination with cellular energy status to ensure an ample energy supply. Our recent work shows that activity of the AMP-activated protein kinase (AMPK), the bio-energy sensor responsible for maintaining cellular energy homeostasis in all eukaryotic cells, plays an important role in the initiation of axonal growth. AMPK phosphorylates the cargo-binding light chain of the Kif5 motor protein, leading to dissociation of the phosphatidylinositol 3-Kinase (PI3K) from the motor complex. The mislocation of PI3K, which is normally enriched at the axonal tip for extension and differentiation, results in a lack of neurite specification and neuron polarization. These findings reveal a link between cellular bioenergy homeostasis and neuron morphogenesis, and suggest a novel cellular mechanism underlying the long-term neurological abnormalities as a consequence of bioenergy deficiency during early brain development.
Keywords: :
Underlying biological functions is the ability of the cell to drive processes against their equilibrium. In evolving this ability, cells posses the capacity to adapt and thrive under staggeringly varied conditions and orchestrate multicellular life. However, driving thermodynamically unfavorable reactions does not come easily and requires a significant input of energy. In the case of the cell, energetic input is achieved by the hydrolysis of adenosine triphosphate (ATP), a molecule used for the storage and transport of cellular energy. In order for this system to function efficiently, a reliable energy supply must exist, enabling the cell to instantly respond to extracellular stimuli. In light of this, eukaryotic cells have evolved AMP-activated protein kinase (AMPK). Originally identified as a protein activated by adenosine monophosphate (AMP) and capable of inactivating enzymes involved in lipid synthesis, AMPK has emerged as a major regulator of energy homeostasis in all eukaryotic cells.Citation1
AMPK is activated in response to metabolic and environmental stresses that deplete ATP and functions in energy homeostasis by inhibiting ATP consuming anabolic processes, while simultaneously promoting ATP generating catabolic processes to produce ATP. While common targets of AMPK include enzymes of major metabolic processes, such as glycolysis and fatty acid oxidation,Citation2 AMPK is also capable of affecting the transcription of proteins involved in energy production and consumption through phosphorylation of various transcription factors,Citation3 thereby enabling AMPK to exert long-term effects on energy expenditure.
AMPK Structure and Activation
AMPK is a heterotrimeric protein that consists of an α, β and γ subunit in equal stoichiometry. The α subunit constitutes the catalytic domain, conferring kinase activity, while the γ subunit enables AMPK to monitor cellular energy status through two AMP/ATP binding domains, referred to as Bateman domains, that bind AMP or ATP in a mutually exclusive manner.Citation4-Citation6 The hydrolysis of ATP, needed for driving thermodynamically unfavorable processes, results in the formation of adendosine monophosphate (AMP). Therefore, high concentrations of AMP serve as a signal of increased energy expenditure and promote the binding of AMP to the Bateman domains. AMP binding causes AMPK to undergo a conformational change, exposing an activation loop of the α subunit, allowing phosphorylation on Threonine172 by upstream kinases, referred to as AMPK kinases (AMPKKs), resulting in a 50–100-fold increase in the catalytic activity of AMPK.Citation7 Conversely, a high concentration of intracellular ATP, characteristic of ample energy reserves, promotes ATP/Bateman domain binding and produces an antagonistic effect on AMPK activation.Citation1,Citation8
AMPK in Neuronal Polarization
Unlike other eukaryotic cell types, neurons must undergo a process of morphological polarization in order to function properly. This process entails the selection and differentiation of multiple dendrites and an axon from a single cell body, which is required for neurons to send and receive information, providing the foundation for the function of neural networks. Neuronal polarization begins with the rapid extension of a single minor neurite into an axon of remarkable size and complex geometry, while the remaining sister neurites will eventually develop into dendrites.Citation9-Citation11 The scale of biosynthesis required for axonal growth and thereby polarization, necessitates increased protein and membrane synthesis, in addition to the intracellular delivery of these building blocks to the growing axon tip.Citation12 Considering the degree of ATP consumption involved in axon formation, AMPK is likely to be involved in the regulation of neuronal polarization.
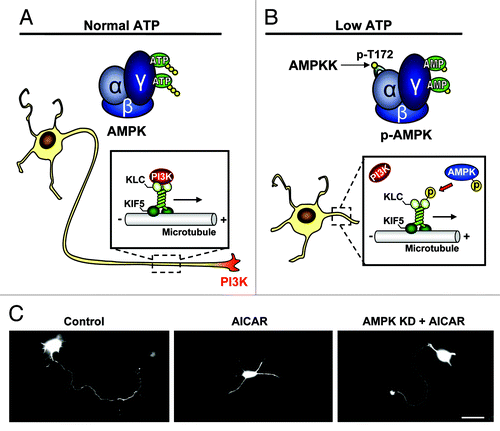
Our recent study has demonstrated that pharmacological activation of AMPK, mimicking energy lacking conditions, during the transition from the symmetric stage 2 to the polarizing stage 3, where the initial signals for axon specification are starting to occur,Citation13 effectively inhibits axon specification and neuronal polarization in both cultured embryonic hippocampal neurons and embryonic cortical brain slices.Citation14 Mechanistically, we show that direct phosphorylation of the kinesin light chain of the motor protein, Kif5, results in a dissociation between the motor complex and its PI3K cargo, thereby preventing PI3K enrichment at the neurite tip, a key mechanism in axon selection and growthCitation9,Citation13 (). Importantly, expression of a kinase dead AMPK mutant (AMPK KD) can rescue polarity in cultured hippocampal neurons and cortical brain slices, regardless of AICAR treatment, indicating that AMPK upregulation, but not its basal activity, regulates neuron polarization ().Citation14 Consistent with this note, a recent study by Williams et al. has shown that genetic knockout of both AMPK α1/α2 catalytic isoforms in mice had no effect on cortical neurogenesis or polarization.Citation15 To indicate clinical significance, we find that brief ischemia challenge during neuronal development causes phosphorylation of AMPK and inhibition of neuronal polarization in cultured hippocampal neurons.Citation14 Similarly, expression of AMPK KD successfully rescued polarity in ischemia treated neurons, concluding that ischemia induced polarity inhibition is directly mediated by AMPK.
Figure 1. Schematic illustration depicting the mechanism of AMPK dependent polarity inhibition. (A) Under normal energy conditions AMPK exists in an unphosphorylated/inactive state and PI3K is transported to the neurite tip via a physical association with the kif5 cargo adaptor, KLC. The accumulation of PI3K at a single neurite tip promotes the signaling responsible for axon initiation and growth. (B) Under energy-lacking conditions, AMP binds to AMPK producing a conformational change in the kinase, allowing phospho-activation of AMPK by upstream kinases (AMPKK). AMPK-caused KLC phosphorylation dissociates PI3K, resulting in a loss of PI3K from the neurite tip and an inhibition of neuronal polarization. (C) Cultured hippocampal neurons are transfected with GFP for visualization. Control neuron shows typical single axon (left), which is missing in a neuron treated with AMPK activator AICAR (middle). Expression of kinase dead (KD) AMPK rescues polarity in the AICAR treated neuron. Scale bar = 20 μm.
Intriguing to this work is the fact that the serine/threonine kinase, LKB1, the major upstream activator of AMPK in peripheral cells,Citation16-Citation20 is required for successful polarization of neurons.Citation21,Citation22 However, despite the fact that both LKB1 and AMPK are expressed in nervous tissue,Citation21,Citation23 studies have indicated that LKB1 may not be the major regulator of AMPK phosphorylation within the brain. For example, under basal conditions, LKB1-deficient cortical neurons show no deficit in phosphorylated AMPK when compared with wild-type cells,Citation21 indicating an alternative means of AMPK phosphorylation. However, it is possible that regulation of AMPK by LKB1 is restricted to periods of energetic stress, when AMP binding has exposed the activation loop of AMPK. It will be interesting to know whether AMPK can be phosphorylated in LKB1-deficient neurons under ATP-lacking conditions. In line with this, AMPK has indeed been suggested to be activated by other upstream signaling. Hawley et al. has shown that in rat brain slices, increases of intracellular calcium by membrane depolarization result in AMPK phosphorylation.Citation24 Phosphorylation of AMPK was concluded to result from CaMKK activity, as the CaMKK inhibitor, STO-609, abolished the effect. Importantly, membrane depolarization did not alter intracellular AMP ratios, indicating that AMPK can be regulated in a Ca2+-dependent, AMP-independent manner. Considering that Ca2+ influx is a hallmark of post-synaptic receptor activation, the ability of AMPK to be phosphorylated by CaMKK may present a mechanism for coupling synaptic activity with energy regulation.
AMPK in Neuronal Glucose Uptake
Despite the ability of AMPK to inhibit PI3K localization at the neurite tip, AICAR treatment causes a marked increase in phosphorylated Akt.Citation14 This effect results directly from AMPK activation, as introduction of the AMPK antagonist successfully blocks AICAR-induced Akt phosphorylation. Furthermore, addition of a PI3K inhibitor also abolishes AICAR-induced Akt phosphorylation, indicating that the AMPK effect on Akt activation is mediated via PI3K.Citation14 Although the physiological role of AMPK-dependent PI3K/Akt activation remains to be investigated, we hypothesize that it may represent a mechanism for stimulating ATP production. Specifically, AMPK has previously been characterized as an upstream regulator of glucose uptake in neurons, a mechanism that involves increased translocation of the glucose transporter, GLUT3, to the surface membrane.Citation25 Similarly, insulin-like growth factor 1 (IGF1) has also been implicated in the regulation of glucose uptake in the brainCitation26-Citation29 and binds to receptor tyrosine kinases that associate with insulin receptor substrate (IRS-1), a known upstream activator of the PI3K/Akt signaling cascade.Citation30,Citation31 Interestingly, AMPK has been observed to phosphorylate IRS-1, the most upstream component in the PI3K signaling pathway, in cell free assays and mouse myoblast C2C12 cell lines in response to AICAR treatment,Citation32 suggesting IRS-1 as the intermediate factor linking AMPK to PI3K/Akt activation.
Clinical Significance
The ability of AMPK to suppress neuronal polarization may have significant relevance within a clinical context. Since neuron polarization and appropriate axon extension are the necessary precondition for intercellular connection and communication, the disruption of neuronal polarity resulting from AMPK activation should have long-lasting effects on synapse formation and ultimately, brain function. We find that AICAR treated neurons fail to specify an axon even after 3 d of recovery following treatment, indicating that the AMPK effect on neuronal polarization may persist even after energy levels return to normal. In a similar scenario, pathological challenges occurring in early development, such as neonatal stroke and hypoxic-ischemic encephalopathy, that have been observed to promote AMPK activation,Citation33-Citation35 also produce late-emergence cognitive deficits.Citation36 It will be interesting to know whether AMPK signaling is indeed responsible for clinically observed neurological abnormalities related to neural energy deficiency during early development.
References
- Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol 2007; 8:774 - 85; http://dx.doi.org/10.1038/nrm2249; PMID: 17712357
- Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab 2005; 1:15 - 25; http://dx.doi.org/10.1016/j.cmet.2004.12.003; PMID: 16054041
- Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 2007; 282:30107 - 19; http://dx.doi.org/10.1074/jbc.M705325200; PMID: 17711846
- Bateman A. The structure of a domain common to archaebacteria and the homocystinuria disease protein. Trends Biochem Sci 1997; 22:12 - 3; http://dx.doi.org/10.1016/S0968-0004(96)30046-7; PMID: 9020585
- Scott JW, Hawley SA, Green KA, Anis M, Stewart G, Scullion GA, et al. CBS domains form energy-sensing modules whose binding of adenosine ligands is disrupted by disease mutations. J Clin Invest 2004; 113:274 - 84; PMID: 14722619
- Xiao B, Heath R, Saiu P, Leiper FC, Leone P, Jing C, et al. Structural basis for AMP binding to mammalian AMP-activated protein kinase. Nature 2007; 449:496 - 500; http://dx.doi.org/10.1038/nature06161; PMID: 17851531
- Hawley SA, Davison M, Woods A, Davies SP, Beri RK, Carling D, et al. Characterization of the AMP-activated protein kinase kinase from rat liver and identification of threonine 172 as the major site at which it phosphorylates AMP-activated protein kinase. J Biol Chem 1996; 271:27879 - 87; http://dx.doi.org/10.1074/jbc.271.44.27879; PMID: 8910387
- Cheung PC, Salt IP, Davies SP, Hardie DG, Carling D. Characterization of AMP-activated protein kinase gamma-subunit isoforms and their role in AMP binding. Biochem J 2000; 346:659 - 69; http://dx.doi.org/10.1042/0264-6021:3460659; PMID: 10698692
- Arimura N, Kaibuchi K. Neuronal polarity: from extracellular signals to intracellular mechanisms. Nat Rev Neurosci 2007; 8:194 - 205; http://dx.doi.org/10.1038/nrn2056; PMID: 17311006
- Craig AM, Banker G. Neuronal polarity. Annu Rev Neurosci 1994; 17:267 - 310; http://dx.doi.org/10.1146/annurev.ne.17.030194.001411; PMID: 8210176
- Ropireddy D, Scorcioni R, Lasher B, Buzsáki G, Ascoli GA. Axonal morphometry of hippocampal pyramidal neurons semi-automatically reconstructed after in vivo labeling in different CA3 locations. Brain Struct Funct 2011; 216:1 - 15; http://dx.doi.org/10.1007/s00429-010-0291-8; PMID: 21128083
- Jareb M, Banker G. Inhibition of axonal growth by brefeldin A in hippocampal neurons in culture. J Neurosci 1997; 17:8955 - 63; PMID: 9364043
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 2003; 112:63 - 75; http://dx.doi.org/10.1016/S0092-8674(02)01249-7; PMID: 12526794
- Amato S, Liu X, Zheng B, Cantley L, Rakic P, Man HY. AMP-activated protein kinase regulates neuronal polarization by interfering with PI 3-kinase localization. Science 2011; 332:247 - 51; http://dx.doi.org/10.1126/science.1201678; PMID: 21436401
- Williams T, Courchet J, Viollet B, Brenman JE, Polleux F. AMP-activated protein kinase (AMPK) activity is not required for neuronal development but regulates axogenesis during metabolic stress. Proc Natl Acad Sci U S A 2011; 108:5849 - 54; http://dx.doi.org/10.1073/pnas.1013660108; PMID: 21436046
- Hawley SA, Boudeau J, Reid JL, Mustard KJ, Udd L, Mäkelä TP, et al. Complexes between the LKB1 tumor suppressor, STRAD alpha/beta and MO25 alpha/beta are upstream kinases in the AMP-activated protein kinase cascade. J Biol 2003; 2:28; http://dx.doi.org/10.1186/1475-4924-2-28; PMID: 14511394
- Sakamoto K, McCarthy A, Smith D, Green KA, Grahame Hardie D, Ashworth A, et al. Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J 2005; 24:1810 - 20; http://dx.doi.org/10.1038/sj.emboj.7600667; PMID: 15889149
- Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, et al. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc Natl Acad Sci U S A 2004; 101:3329 - 35; http://dx.doi.org/10.1073/pnas.0308061100; PMID: 14985505
- Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, et al. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 2005; 310:1642 - 6; http://dx.doi.org/10.1126/science.1120781; PMID: 16308421
- Woods A, Johnstone SR, Dickerson K, Leiper FC, Fryer LG, Neumann D, et al. LKB1 is the upstream kinase in the AMP-activated protein kinase cascade. Curr Biol 2003; 13:2004 - 8; http://dx.doi.org/10.1016/j.cub.2003.10.031; PMID: 14614828
- Barnes AP, Lilley BN, Pan YA, Plummer LJ, Powell AW, Raines AN, et al. LKB1 and SAD kinases define a pathway required for the polarization of cortical neurons. Cell 2007; 129:549 - 63; http://dx.doi.org/10.1016/j.cell.2007.03.025; PMID: 17482548
- Shelly M, Cancedda L, Heilshorn S, Sumbre G, Poo MM. LKB1/STRAD promotes axon initiation during neuronal polarization. Cell 2007; 129:565 - 77; http://dx.doi.org/10.1016/j.cell.2007.04.012; PMID: 17482549
- Turnley AM, Stapleton D, Mann RJ, Witters LA, Kemp BE, Bartlett PF. Cellular distribution and developmental expression of AMP-activated protein kinase isoforms in mouse central nervous system. J Neurochem 1999; 72:1707 - 16; http://dx.doi.org/10.1046/j.1471-4159.1999.721707.x; PMID: 10098881
- Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, et al. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab 2005; 2:9 - 19; http://dx.doi.org/10.1016/j.cmet.2005.05.009; PMID: 16054095
- Weisová P, Concannon CG, Devocelle M, Prehn JH, Ward MW. Regulation of glucose transporter 3 surface expression by the AMP-activated protein kinase mediates tolerance to glutamate excitation in neurons. J Neurosci 2009; 29:2997 - 3008; http://dx.doi.org/10.1523/JNEUROSCI.0354-09.2009; PMID: 19261894
- Bartlett GR. Phosphorus assay in column chromatography. J Biol Chem 1959; 234:466 - 8; PMID: 13641241
- Bondy CA. Transient IGF-I gene expression during the maturation of functionally related central projection neurons. J Neurosci 1991; 11:3442 - 55; PMID: 1658250
- Cheng CM, Reinhardt RR, Lee WH, Joncas G, Patel SC, Bondy CA. Insulin-like growth factor 1 regulates developing brain glucose metabolism. Proc Natl Acad Sci U S A 2000; 97:10236 - 41; http://dx.doi.org/10.1073/pnas.170008497; PMID: 10954733
- Reinhardt RR, Bondy CA. Insulin-like growth factors cross the blood-brain barrier. Endocrinology 1994; 135:1753 - 61; http://dx.doi.org/10.1210/en.135.5.1753; PMID: 7525251
- Myers MG Jr., Sun XJ, White MF. The IRS-1 signaling system. Trends Biochem Sci 1994; 19:289 - 93; http://dx.doi.org/10.1016/0968-0004(94)90007-8; PMID: 8048169
- Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol 2002; 12:65 - 71; http://dx.doi.org/10.1016/S0962-8924(01)02207-3; PMID: 11849969
- Jakobsen SN, Hardie DG, Morrice N, Tornqvist HE. 5′-AMP-activated protein kinase phosphorylates IRS-1 on Ser-789 in mouse C2C12 myotubes in response to 5-aminoimidazole-4-carboxamide riboside. J Biol Chem 2001; 276:46912 - 6; http://dx.doi.org/10.1074/jbc.C100483200; PMID: 11598104
- Li J, Coven DL, Miller EJ, Hu X, Young ME, Carling D, et al. Activation of AMPK alpha- and gamma-isoform complexes in the intact ischemic rat heart. Am J Physiol Heart Circ Physiol 2006; 291:H1927 - 34; http://dx.doi.org/10.1152/ajpheart.00251.2006; PMID: 16648175
- Mungai PT, Waypa GB, Jairaman A, Prakriya M, Dokic D, Ball MK, et al. Hypoxia triggers AMPK activation through reactive oxygen species-mediated activation of calcium release-activated calcium channels. Mol Cell Biol 2011; 31:3531 - 45; http://dx.doi.org/10.1128/MCB.05124-11; PMID: 21670147
- Papandreou I, Lim AL, Laderoute K, Denko NC. Hypoxia signals autophagy in tumor cells via AMPK activity, independent of HIF-1, BNIP3, and BNIP3L. Cell Death Differ 2008; 15:1572 - 81; http://dx.doi.org/10.1038/cdd.2008.84; PMID: 18551130
- Westmacott R, MacGregor D, Askalan R, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke 2009; 40:2012 - 9; http://dx.doi.org/10.1161/STROKEAHA.108.533976; PMID: 19423855