Abstract
Kainate receptors (KARs) are tetrameric glutamate-gated ion channels composed of combinations of the subunits GluK1-5. Depending on their precise localization and subunit composition, KARs can regulate neurotransmitter release, synaptic function and neuronal excitability. Because of these diverse roles, the regulated and precisely targeted trafficking of KARs is of crucial importance to neuronal function. We previously reported that the KAR subunit GluK2 is post-translationally modified by attachment of Small Ubiquitin-like Modifier 1 (SUMO-1) and that SUMOylation is required for agonist-dependent endocytosis of GluK2. We recently extended these findings to demonstrate that agonist activation leads to PKC-mediated phosphorylation of GluK2 at serine 868, which directly enhances GluK2 SUMOylation and, in turn, leads to endocytosis of the receptor. These new data demonstrate the importance of interplay between two post-translational modifications in orchestrating the temporal and spatial regulation of kainate receptor trafficking.
The covalent attachment of a member of the SUMO family to lysine residues in target proteins occurs via an enzymatic cascade analogous to, but distinct from, that of the ubiquitin system. SUMOylation occurs via a three step pathway comprising an E1, a heterodimer of SAE1 and SAE2; a sole E2, Ubc9; and a growing number of identified E3 enzymes.Citation1 Intriguingly, multiple synaptic proteins have been proposed to be, or have been confirmed as, SUMO substrates and protein SUMOylation is strongly implicated in a range of neurodegenerative disorders, suggesting a fundamental role for protein SUMOylation in regulating neuronal functionCitation2 and dysfunction.Citation3,Citation4
Ischemia and other forms of cell stress can enhance global protein SUMOylationCitation5-Citation7 but the SUMOylation of individual proteins is regulated in a substrate-specific manner. Therefore, a major unresolved question for the majority of SUMO substrates is exactly how SUMOylation is initiated and regulated. One way that this may be achieved is by the coordinated interplay between post-translational modifications at specific protein targets. For example, SUMOylation can interfere with, or facilitate other lysine-based modifications such as ubiquitination or acetylation, leading to complex cross-regulation between these systems.Citation7,Citation8 Furthermore, substrate phosphorylation has been reported to either enhance or inhibit SUMOylation, depending on the target protein.Citation1
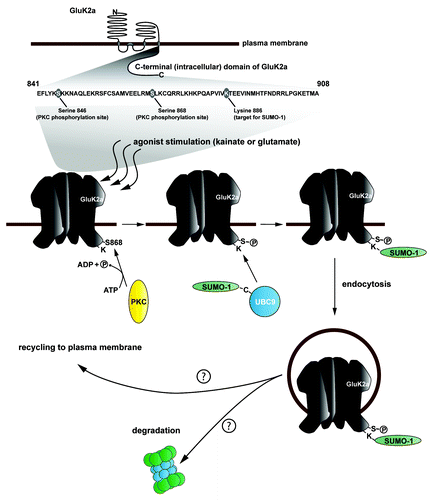
As for most, if not all, other neurotransmitter receptors, it is well-established that KAR function can be regulated by phosphorylation.Citation9-Citation15 It is less clear, however, where and how direct phosphorylation of KAR subunits affects receptor trafficking, localization and function. We therefore investigated if GluK2 itself is phosphorylated following agonist-stimulation and, if so, how this impacts upon GluK2 SUMOylation.Citation16 To do this we first confirmed that virally-expressed GluK2 is robustly phosphorylated in neurons in response to kainate stimulation. This phosphorylation was reduced by the protein kinase C (PKC) inhibitor chelerythrine. In addition, in agreement with another recent study of GluK2 phosphorylation,Citation17 mutation of either serine 846 or serine 868 also reduced kainate-evoked phosphorylation of GluK2. Interestingly, some agonist-induced phosphorylation of GluK2 remained after incubation with chelerythrine, raising the possibility that other, as yet undefined, kinases also play a role in regulating GluK2 trafficking. We next examined whether modulating PKC activity regulated GluK2 SUMOylation. Consistent with SUMOylation being downstream of phosphorylation we found that activating PKC with phorbol ester enhanced GluK2 SUMOylation. Taken together, these results indicate that PKC phosphorylation is a requirement for agonist-induced SUMOylation of GluK2.
Importantly, we confirmed that this effect of PKC phosphorylation is direct by demonstrating that in vitro phosphorylation of the C-terminus of GluK2 by PKC enhances its subsequent in vitro SUMOylation. Phosphomimetic mutation of the PKC site S868 enhanced GluK2 SUMOylation in COS-7 cells whereas phosphomimetic mutation of S846 did not, indicating that the facilitation of SUMOylation is site-specific. Thus, PKC phosphorylates both S846 and S868 but only phosphorylation of S868 acts to enhance receptor SUMOylation. Although S846 has been implicated in the regulation of basal GluK2 endocytosisCitation17 it does not affect agonist-induced receptor endocytosis.Citation16
In reciprocal experiments, S868 was mutated to a non-phosphorylatable alanine residue. Following expression in neurons wild-type GluK2 was SUMOylated in response to kainate, however this effect was lost for the S868A mutant. These results confirm that prior PKC phosphorylation at S868 is necessary for receptor SUMOylation in response to kainate. Further, in contrast to wild-type GluK2, the S868 mutant did not undergo agonist-induced endocytosis. Taken together, our findings suggest a model whereby agonist simulation of GluK2-containing KARs leads to activation of PKC and direct phosphorylation of GluK2 at S846 and S868. While the role of agonist-induced S846 phosphorylation remains to be fully elucidated, phosphorylation of S868 is required for the increased SUMOylation and consequent endocytosis of the receptor ().
Figure 1. Schematic illustrating the roles of PKC phosphorylation and SUMOylation on the trafficking of GluK2-containing KARs. The top panel represents the membrane topology of GluK2, highlighting the intracellular C-terminus where PKC phosphorylation and SUMOylation occur. The sequence of this region is shown and the modified amino acids indicated. Agonist activation of GluK2 leads to PKC-mediated phosphorylation at S868 (as well as S846, not shown), which directly leads to receptor SUMOylation and endocytosis. Once internalized, receptors are subject to post-endocytic sorting either into recycling pool and exocytosed back to the neuronal plasma membrane or they are targeted for degradation.
These findings shed new light on the regulatory mechanisms and complex interactions involved in KAR trafficking. This is important because the processes that orchestrate KAR availability, localization and function determine the responsiveness and viability of neurons, and also profoundly influence neuronal network properties. More generally, our results confirm and extend the importance of the interplay between SUMOylation and phosphorylation in dictating the spatial and temporal specificity of substrate SUMOylation.
Of course, many intriguing questions remain to be addressed. For example, kainate application causes activation of PKC but exactly how this occurs is unclear. Further, while we have demonstrated that PKC phosphorylation of GluK2 enhances SUMOylation in vitro, the underlying mechanism remains to be determined. The in vitro assay contains only the E1 and E2 SUMOylation enzymes, suggesting that phosphorylation likely acts to recruit one (or both) of these components to GluK2. Substrate phosphorylation has previously been reported to recruit the SUMO E2 enzyme Ubc9, through the binding of a cognate basic patch on Ubc9.Citation18-Citation20 While this remains possible for GluK2, the PKC phosphorylation site at S868 is relatively distant from the SUMOylated lysine (K886) in comparison to other substrates in which SUMOylation is increased through phospho-mediated recruitment of Ubc9. Thus, the direct mechanism of the phosphorylation-mediated enhancement of GluK2 SUMOylation is another important question that awaits an answer. While this study reaffirms the crucial role of GluK2 SUMOylation in regulating KAR endocytosis, perhaps the most pressing outstanding question is how this process works. SUMOylation may, for example, recruit proteins of the endocytic machinery, or act to disrupt the anchoring of GluK2 at the neuronal membrane. We believe that future work aimed at directly addressing these questions will deepen our understanding of both physiological and pathophysiological KAR trafficking and function, and could reveal potential for either KARs or the SUMOylation pathway as therapeutic targets in a number of disorders of the nervous system.
Acknowledgments
We are grateful to the MRC, BBSRC, Wellcome Trust and ERC for financial support and to all of the authors on the Proceedings of the National Academy of Sciences of the United States of America paper for their invaluable contributions.
References
- Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J 2010; 428:133 - 45; http://dx.doi.org/10.1042/BJ20100158; PMID: 20462400
- Wilkinson KA, Nakamura Y, Henley JM. Targets and consequences of protein SUMOylation in neurons. Brain Res Rev 2010; 64:195 - 212; http://dx.doi.org/10.1016/j.brainresrev.2010.04.002; PMID: 20382182
- Anderson DB, Wilkinson KA, Henley JM. Protein SUMOylation in neuropathological conditions. Drug News Perspect 2009; 22:255 - 65; http://dx.doi.org/10.1358/dnp.2009.22.5.1378636; PMID: 19609463
- Dorval V, Fraser PE. SUMO on the road to neurodegeneration. Biochim Biophys Acta 2007; 1773:694 - 706; http://dx.doi.org/10.1016/j.bbamcr.2007.03.017; PMID: 17475350
- Agbor TA, Taylor CT. SUMO, hypoxia and the regulation of metabolism. Biochem Soc Trans 2008; 36:445 - 8; http://dx.doi.org/10.1042/BST0360445; PMID: 18481978
- Tempé D, Piechaczyk M, Bossis G. SUMO under stress. Biochem Soc Trans 2008; 36:874 - 8; http://dx.doi.org/10.1042/BST0360874; PMID: 18793154
- Bossis G, Melchior F. SUMO: regulating the regulator. Cell Div 2006; 1:13; http://dx.doi.org/10.1186/1747-1028-1-13; PMID: 16805918
- Geoffroy MC, Hay RT. An additional role for SUMO in ubiquitin-mediated proteolysis. Nat Rev Mol Cell Biol 2009; 10:564 - 8; http://dx.doi.org/10.1038/nrm2707; PMID: 19474794
- Martin S, Nishimune A, Mellor JR, Henley JM. SUMOylation regulates kainate-receptor-mediated synaptic transmission. Nature 2007; 447:321 - 5; http://dx.doi.org/10.1038/nature05736; PMID: 17486098
- Park Y, Jo J, Isaac JT, Cho K. Long-term depression of kainate receptor-mediated synaptic transmission. Neuron 2006; 49:95 - 106; http://dx.doi.org/10.1016/j.neuron.2005.11.035; PMID: 16387642
- Selak S, Paternain AV, Aller MI, Picó E, Rivera R, Lerma J. A role for SNAP25 in internalization of kainate receptors and synaptic plasticity. Neuron 2009; 63:357 - 71; http://dx.doi.org/10.1016/j.neuron.2009.07.017; PMID: 19679075
- Cho K, Francis JC, Hirbec H, Dev K, Brown MW, Henley JM, et al. Regulation of kainate receptors by protein kinase C and metabotropic glutamate receptors. J Physiol 2003; 548:723 - 30; http://dx.doi.org/10.1113/jphysiol.2003.040188; PMID: 12640005
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, et al. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 2003; 37:625 - 38; http://dx.doi.org/10.1016/S0896-6273(02)01191-1; PMID: 12597860
- Rivera R, Rozas JL, Lerma J. PKC-dependent autoregulation of membrane kainate receptors. EMBO J 2007; 26:4359 - 67; http://dx.doi.org/10.1038/sj.emboj.7601865; PMID: 17898803
- Martin S, Henley JM. Activity-dependent endocytic sorting of kainate receptors to recycling or degradation pathways. EMBO J 2004; 23:4749 - 59; http://dx.doi.org/10.1038/sj.emboj.7600483; PMID: 15549132
- Konopacki FA, Jaafari N, Rocca DL, Wilkinson KA, Chamberlain S, Rubin P, et al. Agonist-induced PKC phosphorylation regulates GluK2 SUMOylation and kainate receptor endocytosis. Proc Natl Acad Sci U S A 2011; 108:19772 - 7; http://dx.doi.org/10.1073/pnas.1111575108; PMID: 22089239
- Nasu-Nishimura Y, Jaffe H, Isaac JT, Roche KW. Differential regulation of kainate receptor trafficking by phosphorylation of distinct sites on GluR6. J Biol Chem 2010; 285:2847 - 56; http://dx.doi.org/10.1074/jbc.M109.081141; PMID: 19920140
- Yang SH, Galanis A, Witty J, Sharrocks AD. An extended consensus motif enhances the specificity of substrate modification by SUMO. EMBO J 2006; 25:5083 - 93; http://dx.doi.org/10.1038/sj.emboj.7601383; PMID: 17036045
- Hietakangas V, Anckar J, Blomster HA, Fujimoto M, Palvimo JJ, Nakai A, et al. PDSM, a motif for phosphorylation-dependent SUMO modification. Proc Natl Acad Sci U S A 2006; 103:45 - 50; http://dx.doi.org/10.1073/pnas.0503698102; PMID: 16371476
- Mohideen F, Capili AD, Bilimoria PM, Yamada T, Bonni A, Lima CD. A molecular basis for phosphorylation-dependent SUMO conjugation by the E2 UBC9. Nat Struct Mol Biol 2009; 16:945 - 52; http://dx.doi.org/10.1038/nsmb.1648; PMID: 19684601